In Meniere's disease, diuretics, commonly known as water pills, play a crucial role in managing symptoms associated with fluid retention in the inner ear. Meniere's disease is characterized by a buildup of excess fluid in the inner ear, leading to symptoms such as vertigo, hearing loss, and tinnitus. Diuretics help address this issue by promoting increased urine production, thereby reducing fluid levels in the body, including the inner ear. By facilitating the elimination of excess fluids, diuretics can help alleviate symptoms such as vertigo and restore balance in individuals with Meniere's disease.
Access Full Report at https://www.databridgemarketresearch.com/reports/us-meniere-disease-drug-market
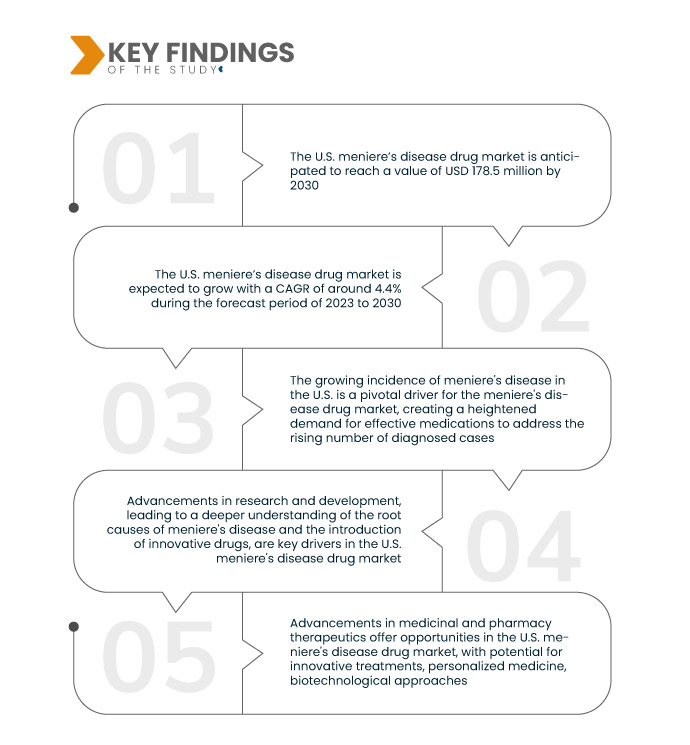
Data Bridge Market Research analyses the U.S. Meniere’s Disease Drug Market, which was USD 126.5 million in 2022, is expected to reach up to USD 178.5 million by 2030 and is expected to undergo a CAGR of 4.4% from 2023 to 2030. Government initiatives and funding in the U.S. for Meniere's disease research and drug development are key drivers of financial support and fostering an environment conducive to innovation.
Key Findings of the Study
Increasing development and enhancement of healthcare infrastructure is expected to drive the market's growth rate
The development and enhancement of healthcare infrastructure in the U.S. play a pivotal role as a driver in the meniere's disease drug market. Improvements in medical facilities, diagnostic technologies, and accessibility enable more efficient diagnosis and treatment of meniere's disease. A well-established healthcare infrastructure ensures that individuals with meniere's disease have timely access to healthcare services, leading to early intervention and increased demand for pharmaceutical solutions. As the healthcare system becomes more adept at addressing meniere's disease, pharmaceutical companies are presented with expanded opportunities for market growth, driven by the synergistic relationship between improved healthcare infrastructure and the rising demand for effective drugs in the U.S. meniere's disease market.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2023 to 2030
|
Base Year
|
2022
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
Segments Covered
|
Type (Classic, Bilateral, Vestibular), Treatment (Drug, Surgical, Suplemental Therapies and Procedure), Drug Type (Generics, Branded), Route of Administration (Oral, Parenteral), End-User (Hospitals, Specialty Clinics, Diagnostic Centers, and Others), Distribution Channel (Direct Tender, Hospital Pharmacies, Retail Pharmacies and Online Pharmacies)
|
Market Players Covered
|
Agouron Pharmaceuticals, LLC (U.S.), AHP Holdings B.V. (Netherlands), Alacer Corp. (U.S.), Alpharma Pharmaceuticals LLC (U.S.), Bioren, LLC (U.S.) and G. D. Searle & Co. Limited (U.K.) Hikma Pharmaceuticals PLC (U.K.), Mylan N.V.(U.S.), Pfizer Inc.(U.S.), Fresenius Kabi AG (Germany), F. Hoffmann-La Roche Ltd. (Switzerland)
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The U.S. meniere's disease drug market is segmented on the basis of type, treatment, drug type, route of administration, distribution channel and end-user.
- On the basis of type, the U.S. meniere's disease drug market is segmented into classic, bilateral, and vestibular
- On the basis of treatment, the meniere's disease drug market is segmented into drug, surgical, suplemental therapies and procedure
- On the basis of drug type, the U.S. meniere's disease drug market is segmented into generics, and branded
- On the basis of route of administration, the U.S. meniere's disease drug market is segmented into oral, intravenous, intratympanic and others
- On the basis of distribution channel, the U.S. meniere's disease drug market is segmented into direct tender, hospital pharmacies, retail pharmacies and online pharmacies
- On the basis of end-user, the U.S. meniere's disease drug market is segmented into hospitals, specialty clinics, diagnostic centers and others
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. meniere’s disease drug market players in U.S. meniere’s disease drug market are Agouron Pharmaceuticals, LLC (U.S.), AHP Holdings B.V. (Netherlands), Alacer Corp. (U.S.), Alpharma Pharmaceuticals LLC (U.S.), Bioren, LLC (U.S.) and G. D. Searle & Co. Limited (U.K.)
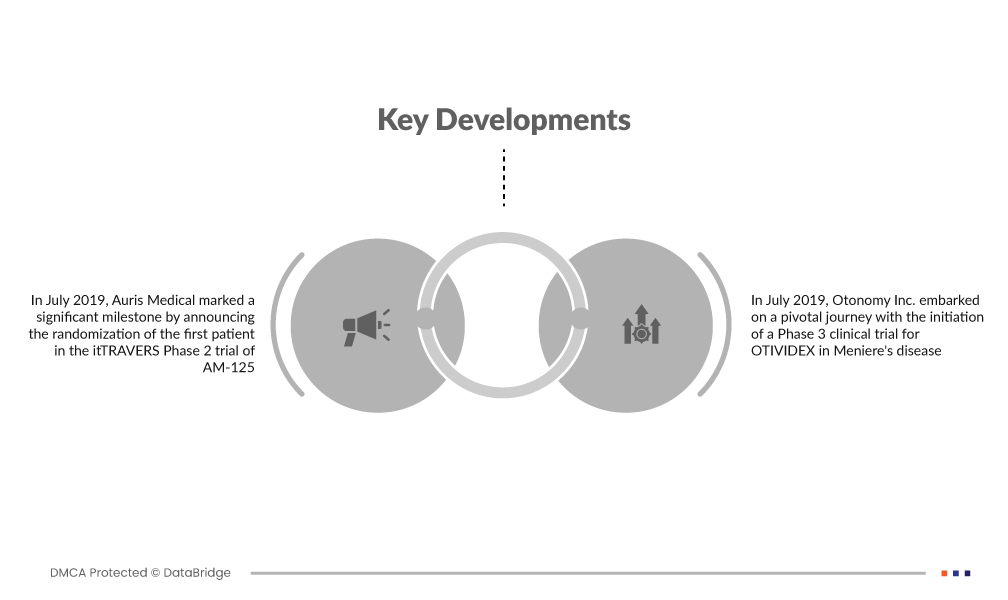
Market Developments
- In July 2019, Auris Medical marked a significant milestone by announcing the randomization of the first patient in the itTRAVERS Phase 2 trial of AM-125. This development underscores the company's commitment to advancing innovative treatments, as AM-125 aims to address unmet medical needs, particularly in the realm of auditory disorders
- In July 2019, Otonomy Inc. embarked on a pivotal journey with the initiation of a Phase 3 clinical trial for OTIVIDEX in Meniere's disease. This strategic move reflects Otonomy's dedication to advancing the clinical development of OTIVIDEX, a potential breakthrough therapy that holds promise in alleviating the symptoms of Meniere's disease and improving the quality of life for affected individuals
For more detailed information about the U.S. meniere’s disease drug market report, click here – https://www.databridgemarketresearch.com/reports/us-meniere-disease-drug-market