Die Palmoplantarpustulose (PPP) kann leichte bis mittelschwere und mittelschwere bis schwere Formen annehmen und betrifft Hände und Füße. Die Diagnose wird durch Laboruntersuchungen auf Entzündungsmarker und eine Hautbiopsie bestätigt. In leichten bis mittelschweren Fällen können lokale Behandlungen und Phototherapie ausreichen. Mittelschwere bis schwere Fälle erfordern oft systemische Medikamente, was die Bedeutung einer präzisen Diagnose unterstreicht. Ein multidisziplinärer Ansatz, der das klinische Erscheinungsbild und die Patientenanamnese berücksichtigt, hilft bei der Entwicklung effektiver Interventionen für Patienten mit unterschiedlich ausgeprägter PPP.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/us-palmoplantar-pustulosis-ppp-market
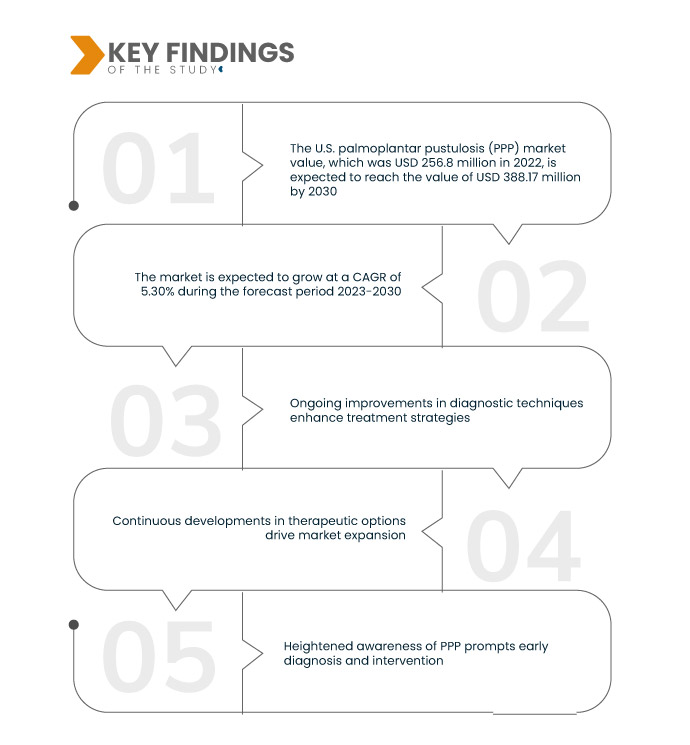
Data Bridge Market Research analysiert, dass der US-Markt für Palmoplantare Pustulose (PPP) von 256,8 Millionen US-Dollar im Jahr 2022 bis 2030 voraussichtlich einen Wert von 388,17 Millionen US-Dollar erreichen wird, was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 5,30 % im Prognosezeitraum 2023–2030 entspricht. Die treibenden multidisziplinären Ansätze unterstreichen die Bedeutung der Integration verschiedener medizinischer Fachrichtungen für eine umfassende Betreuung und Behandlung von Patienten mit Palmoplantarer Pustulose (PPP). Dieser kollaborative Ansatz gewährleistet ein ganzheitliches Verständnis der Erkrankung und führt zu effektiveren und personalisierten Behandlungsstrategien.
Wichtigste Ergebnisse der Studie
Steigende Inzidenz wird voraussichtlich die Wachstumsrate des Marktes antreiben
Der Anstieg der Fälle von Palmoplantarer Pustulose (PPP) treibt das Marktwachstum voran und spiegelt die steigende Inzidenz wider. Da immer mehr Menschen mit PPP diagnostiziert werden, steigt die Nachfrage nach effektiver Diagnostik und Behandlung. Dieser steigende Prävalenztrend erfordert kontinuierliche Forschung und Innovation, um der sich entwickelnden Gesundheitslandschaft im Zusammenhang mit PPP gerecht zu werden. Das Marktwachstum ist eng mit der Notwendigkeit verbunden, den wachsenden Bedürfnissen von Menschen mit dieser dermatologischen Erkrankung gerecht zu werden.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2023 bis 2030
|
Basisjahr
|
2022
|
Historische Jahre
|
2021 (Anpassbar auf 2015–2020)
|
Quantitative Einheiten
|
Umsatz in Millionen USD, Mengen in Einheiten, Preise in USD
|
Abgedeckte Segmente
|
Krankheitstyp (Typ A und Typ B), Schweregrad (leicht-mittelschwer und mittelschwer-schwer), Typ (Diagnose und Behandlung), Bevölkerungstyp (Kinder und Erwachsene), Verabreichungsweg (oral, topisch und parenteral), Endbenutzer (Krankenhäuser, häusliche Pflege, Fachkliniken, andere), Vertriebskanal (Krankenhausapotheke, Online-Apotheke, Einzelhandelsapotheke)
|
Abgedeckte Marktteilnehmer
|
Novartis AG (Schweiz), Lilly (USA), F. Hoffmann-La Roche Ltd. (Schweiz), Bristol-Myers Squibb Company (USA), AstraZeneca (Großbritannien), GSK plc (Großbritannien), Merck & Co., Inc. (USA), Amgen inc. (USA), AnGes, Inc. (USA), Johnson & Johnson Private Limited (USA), AbbVie Inc. (USA), Perrigo Company plc (Irland), WOCKHARDT (Indien), Bausch Health Companies Inc. (Kanada), Viatris Inc. (USA), Amneal Pharmaceuticals LLC. (USA), Xiromed (USA) und Encube Ethicals Private Limited (USA)
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse:
Der US-Markt für Palmoplantarpustulose (PPP) ist nach Krankheitstyp, Schweregrad, Diagnose, Behandlung, Bevölkerungstyp, Verabreichungsweg, Endverbraucher und Vertriebskanal segmentiert.
- Auf der Grundlage des Krankheitstyps wird der US-Markt für Palmoplantarpustulose (PPP) in Typ A und Typ B segmentiert.
- Auf der Grundlage des Schweregrads ist der US-Markt für Palmoplantarpustulose (PPP) in leicht-mittelschwer und mittelschwer-schwer unterteilt
- Auf der Grundlage der Diagnose ist der US-Markt für Palmoplantarpustulose (PPP) in Labortests und Hautbiopsie unterteilt.
- Auf der Grundlage der Behandlung ist der US-Markt für Palmoplantarpustulose (PPP) in Weichmacher, topische Steroide, Kohlenteer und Phototherapie unterteilt
- Auf der Grundlage des Bevölkerungstyps ist der US-Markt für Palmoplantarpustulose (PPP) in Kinder und Erwachsene unterteilt
- Auf der Grundlage der Verabreichungsmethode ist der US-Markt für Palmoplantarpustulose (PPP) in orale, topische und parenterale Verabreichung unterteilt
- Auf der Grundlage des Endverbrauchers ist der US-Markt für Palmoplantarpustulose (PPP) in Krankenhäuser, häusliche Pflege, Fachkliniken und andere unterteilt
- Auf der Grundlage des Vertriebskanals ist der US-Markt für Palmoplantarpustulose (PPP) in Krankenhausapotheke, Online-Apotheke und Einzelhandelsapotheke segmentiert
Hauptakteure
Data Bridge Market Research erkennt die folgenden Unternehmen als Akteure auf dem US-Markt für palmoplantare Pustulose (PPP) an: Novartis AG (Schweiz), Lilly (USA), F. Hoffmann-La Roche Ltd. (Schweiz), Bristol-Myers Squibb Company (USA), AstraZeneca (Großbritannien) und GSK plc (Großbritannien).
Marktentwicklungen
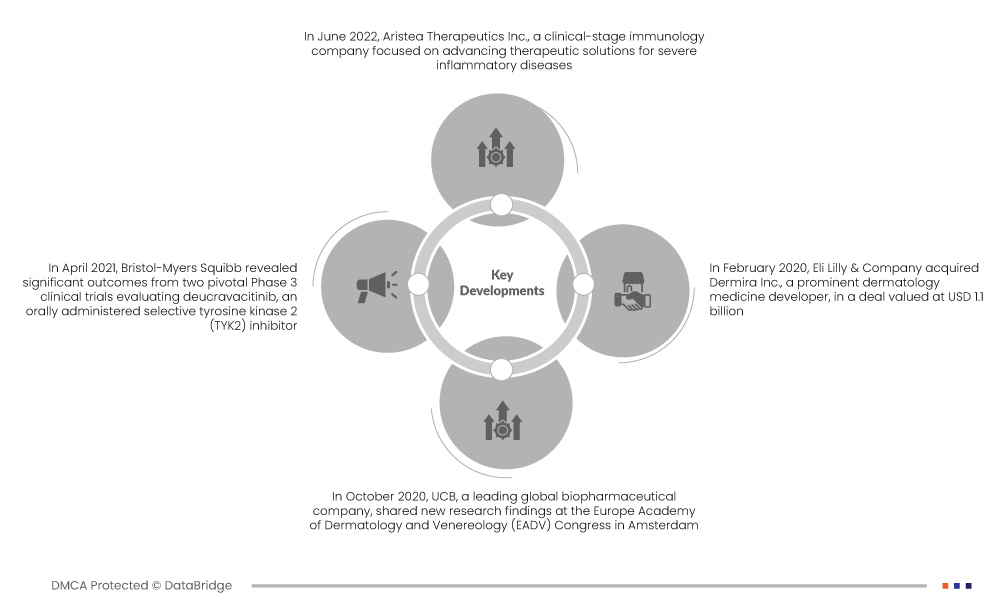
- Im Juni 2022 meldete Aristea Therapeutics Inc., ein Immunologie-Unternehmen im klinischen Stadium, das sich auf die Weiterentwicklung therapeutischer Lösungen für schwere entzündliche Erkrankungen konzentriert, den Beginn der Open-Label-Extension-Phase (OLE) in seiner Phase-2b-Studie mit RIST4721 zur Behandlung der palmoplantaren Pustulose (PPP), einer seltenen Hauterkrankung. Im Rahmen dieser Studie verabreichten sie einem Probanden die erste Dosis.
- Im April 2021 veröffentlichte Bristol-Myers Squibb signifikante Ergebnisse aus zwei zulassungsrelevanten klinischen Phase-3-Studien zur Evaluierung von Deucravacitinib, einem oral verabreichten selektiven Tyrosinkinase-2-(TYK2)-Inhibitor. Ziel dieser Studien war die Bewertung der Wirksamkeit des Medikaments bei der Behandlung von Patienten mit mittelschwerer bis schwerer Plaque-Psoriasis, einer häufigen Autoimmunerkrankung der Haut. Die Ergebnisse unterstrichen das Potenzial von Deucravacitinib als vielversprechende Behandlungsoption für diese Erkrankung und stellten einen Durchbruch in der Psoriasis-Behandlung dar.
- Im Oktober 2020 präsentierte UCB, ein weltweit führendes biopharmazeutisches Unternehmen, neue Forschungsergebnisse auf dem Kongress der Europäischen Akademie für Dermatologie und Venerologie (EADV) in Amsterdam. Die präsentierten Daten konzentrierten sich auf den IL-17A- und IL-17F-Inhibitor Bimekizumab und den TNF-Inhibitor CIMZIA (Certolizumab Pegol) und demonstrierten deren Wirksamkeit bei der Behandlung von Patienten mit mittelschwerer bis schwerer Plaque-Psoriasis. Die Präsentation unterstrich das Potenzial dieser Behandlungen zur Behandlung dieser anspruchsvollen dermatologischen Erkrankung.
- Im Februar 2020 übernahm Eli Lilly & Company Dermira Inc., einen führenden Entwickler dermatologischer Medikamente, im Wert von 1,1 Milliarden US-Dollar. Diese Übernahme erweiterte Eli Lillys Portfolio an dermatologischen Medikamenten deutlich und ermöglichte dem Unternehmen, sein Angebot zu diversifizieren und seine Präsenz auf dem dermatologischen Pharmamarkt zu stärken. Dies war ein strategischer Schritt, um der wachsenden Nachfrage nach dermatologischen Behandlungen und Innovationen in diesem Bereich gerecht zu werden.
Für detailliertere Informationen zum US-Marktbericht zur palmoplantaren Pustulose (PPP) klicken Sie hier – https://www.databridgemarketresearch.com/reports/us-palmoplantar-pustulosis-ppp-market