미국에서는 미세침습녹내장수술(MIGS) 장비 시장이 유망한 녹내장 치료 옵션으로 주목을 받고 있습니다. 녹내장 진행을 막기 위해 안압(IOP)을 낮추는 것이 필수적이기 때문에 역사적으로 수술적 방법과 비수술적 방법이 모두 사용되어 왔지만, 각 방법마다 한계가 있었습니다. MIGS는 특히 경증에서 중등도의 원발성 개방각녹내장에 백내장 수술과 병행할 때 유망한 해결책으로 떠오르고 있습니다. 이처럼 안전하고 효율적인 접근 방식은 기존의 과제를 해결하고 조기 개입 가능성을 제공하며 시장 성장을 촉진합니다.
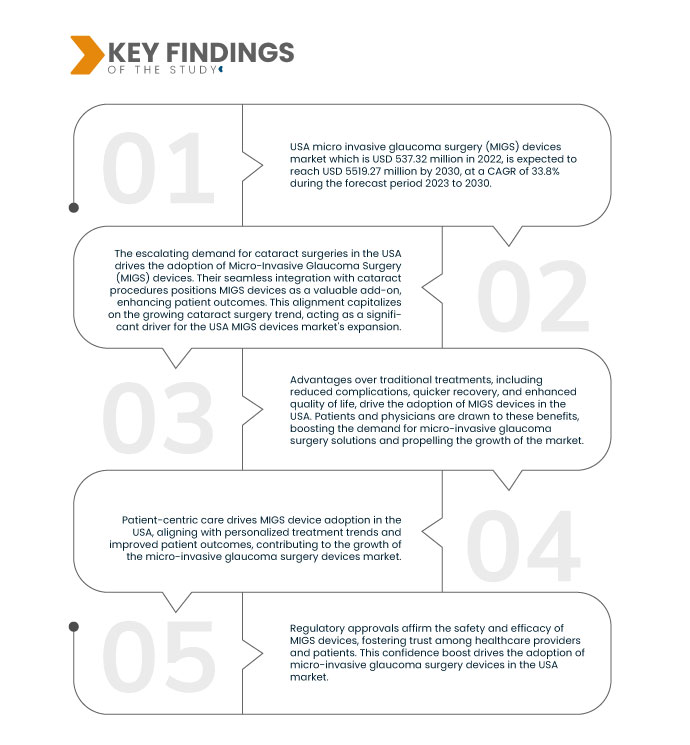
데이터 브리지 마켓 리서치(Data Bridge Market Research)에 따르면 , 2022년 5억 3,732만 달러 규모였던 미국 미세침습녹내장수술(MIGS) 기기 시장은 2030년까지 55억 1,927만 달러에 도달할 것으로 예상되며, 이는 2023년부터 2030년까지 연평균 성장률 33.8%에 달할 것으로 전망됩니다. 외과의들이 MIGS 기기를 선호하는 이유는 우수한 치료 결과와 효율적인 시술 때문입니다. 이러한 성장 동력은 의사들이 효과적이고 안전한 치료 옵션을 선호함에 따라 미국 미세침습녹내장수술(MIGS) 기기 시장의 성장을 촉진하고 있으며, MIGS 기기의 광범위한 도입을 촉진하고 있습니다.
연구의 주요 결과
노인 인구의 확대로 시장 성장률이 높아질 것으로 예상됩니다.
녹내장에 더 취약한 고령 인구 증가는 미세침습 녹내장 수술(MIGS) 기기와 같이 접근성이 높고 환자 중심적인 솔루션에 대한 수요를 촉진합니다. 고령 인구가 편안함과 최소 침습을 중시하는 효과적인 치료법을 추구함에 따라 MIGS 기기의 중요성이 커지고 있습니다. 이러한 성장세는 고령 환자의 고유한 요구를 충족해야 한다는 필요성과 맞닿아 있으며, 미국 MIGS 기기 시장의 성장과 관련성을 가속화합니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2023년부터 2030년까지
|
기준 연도
|
2022
|
역사적인 해
|
2021 (2015-2020년으로 맞춤 설정 가능)
|
양적 단위
|
매출(백만 달러), 볼륨(단위), 가격(달러)
|
다루는 세그먼트
|
제품(MIGS 스텐트, MIGS 션트 및 기타), 대상(트라베큘라 메시워크 상맥락막 공간 결막하 여과 및 방수 생성 감소), 수술 유형(백내장과 동반된 녹내장 및 단독 녹내장), 최종 사용자(병원 외래 진료과(HOPD), 안과 진료소, 외래 수술 센터 (ASCS) 및 기타), 유통 채널(직접 입찰 및 소매 판매)
|
시장 참여자 포함
|
Glaukos Corporation(미국), Ivantis Inc.(미국), AbbVie Inc.(미국), Ellex(미국), Alcon(스위스), BVI(미국), Johnson and Johnson Surgical Vision Care Inc.(미국), Microsurgical technology(미국), Molteno Ophthalmic Ltd.(뉴질랜드), New world Medical Inc.(미국), Santen Pharmaceutical Co., Ltd.(일본), Sight Scientific(미국), Bausch & Lomb Incorporated.(캐나다), NeoMedix Healthcare India Pvt. Ltd.(미국), IOPtima(이스라엘)
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
미세 침습성 녹내장 수술(MIGS) 장치 시장은 대상, 제품, 수술 유형, 유통 채널 및 최종 사용자를 기준으로 세분화됩니다.
- 제품을 기준으로, 미세 침습성 녹내장 수술(MIGS) 장치 시장은 MIGS 스텐트, MIGS 션트 및 기타로 구분됩니다.
- 목표에 따라 미세 침습성 녹내장 수술(MIGS) 장치 시장은 트라베큘라 망상조직, 맥락막상 공간, 결막하 여과, 방수 생성 감소로 세분화됩니다.
- 수술 유형을 기준으로, 미세 침습성 녹내장 수술(MIGS) 장치 시장은 백내장이 동반된 녹내장과 단독 녹내장으로 구분됩니다.
- 최종 사용자를 기준으로, 미세 침습성 녹내장 수술(MIGS) 장치 시장은 병원 외래 환자 부서(HOPD), 안과 진료소, 외래 수술 센터(ASC) 및 기타로 세분화됩니다.
- 유통 채널을 기준으로, 미세 침습성 녹내장 수술(MIGS) 장치 시장은 직접 입찰과 소매 판매로 구분됩니다.
주요 플레이어
Data Bridge Market Research는 다음 회사를 주요 미국 미세 침습성 녹내장 수술(MIGS) 장치 시장 참여자로 인식합니다.미국 미세 침습성 녹내장 수술(MIGS) 장치 시장의 주요 참여자는 Glaukos Corporation(미국), Ivantis Inc.(미국), AbbVie Inc.(미국), Ellex(미국), Alcon(스위스), BVI(미국), Johnson and Johnson Surgical Vision Care Inc.(미국), Microsurgical technology(미국), Molteno Ophthalmic Ltd.(뉴질랜드)입니다.
시장 개발
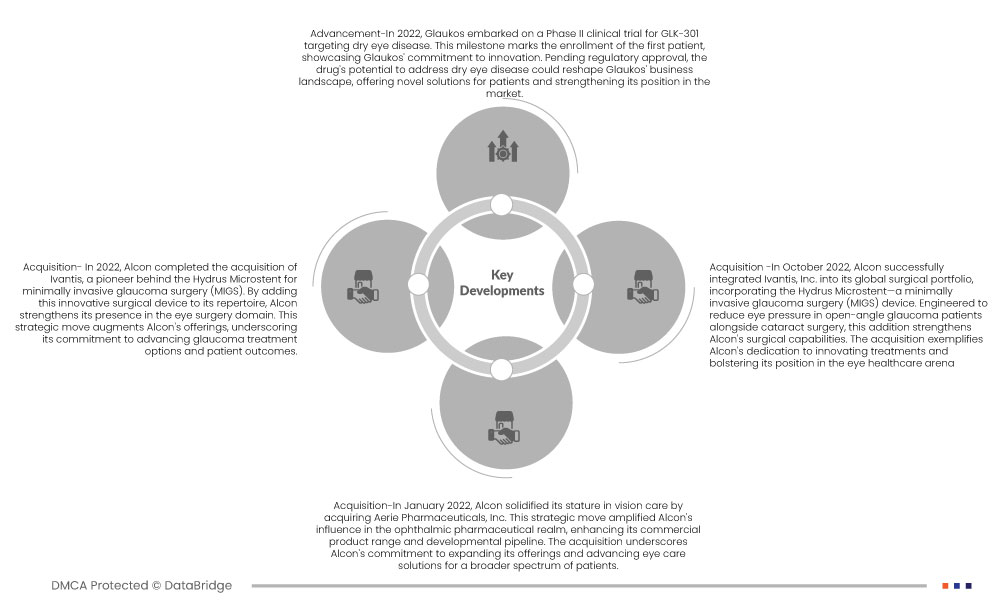
- 2022년, 글라우코스는 안구건조증을 타깃으로 하는 GLK-301의 2상 임상 시험에 착수했습니다. 이 중요한 성과는 첫 환자 등록을 의미하며, 글라우코스의 혁신 의지를 보여줍니다. 규제 당국의 승인을 기다리는 동안, 이 약물이 안구건조증 치료에 기여할 잠재력은 글라우코스의 사업 환경을 재편하고, 환자에게 새로운 솔루션을 제공하며 시장에서의 입지를 강화할 수 있을 것입니다.
- 2022년, 알콘은 최소 침습 녹내장 수술(MIGS)용 하이드러스 마이크로스텐트(Hydrus Microstent)의 선구자인 이반티스(Ivantis)를 인수했습니다. 이 혁신적인 수술 기기를 자사 제품에 추가함으로써 알콘은 안과 수술 분야에서의 입지를 더욱 강화하게 되었습니다. 이러한 전략적 행보는 알콘의 제품 및 서비스를 확대하고, 녹내장 치료 옵션과 환자 치료 결과를 개선하려는 알콘의 노력을 강조합니다.
- 2022년 1월, 알콘은 에이리 파마슈티컬스(Aerie Pharmaceuticals, Inc.)를 인수하여 시력 관리 분야에서의 입지를 더욱 공고히 했습니다. 이러한 전략적 행보는 안과 의약품 분야에서 알콘의 영향력을 확대하여 상용 제품 범위와 개발 파이프라인을 강화했습니다. 이번 인수는 더 광범위한 환자층을 위한 안과 솔루션 제공 및 발전에 대한 알콘의 의지를 보여줍니다.
- 2022년 10월, 알콘(Alcon)은 최소 침습 녹내장 수술(MIGS) 장치인 하이드러스 마이크로스텐트(Hydrus Microstent)를 도입하며 아이반티스(Ivantis, Inc.)를 글로벌 수술 포트폴리오에 성공적으로 통합했습니다. 개방각 녹내장 환자의 안압을 낮추고 백내장 수술을 병행하도록 설계된 이 제품은 알콘의 수술 역량을 강화합니다. 이번 인수는 혁신적인 치료법 개발과 안과 의료 분야에서의 입지 강화에 대한 알콘의 헌신을 보여주는 사례입니다.
미국 미세 침습성 녹내장 수술(MIGS) 장치 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요. - https://www.databridgemarketresearch.com/reports/usa-micro-invasive-glaucoma-surgery-migs-devices-market