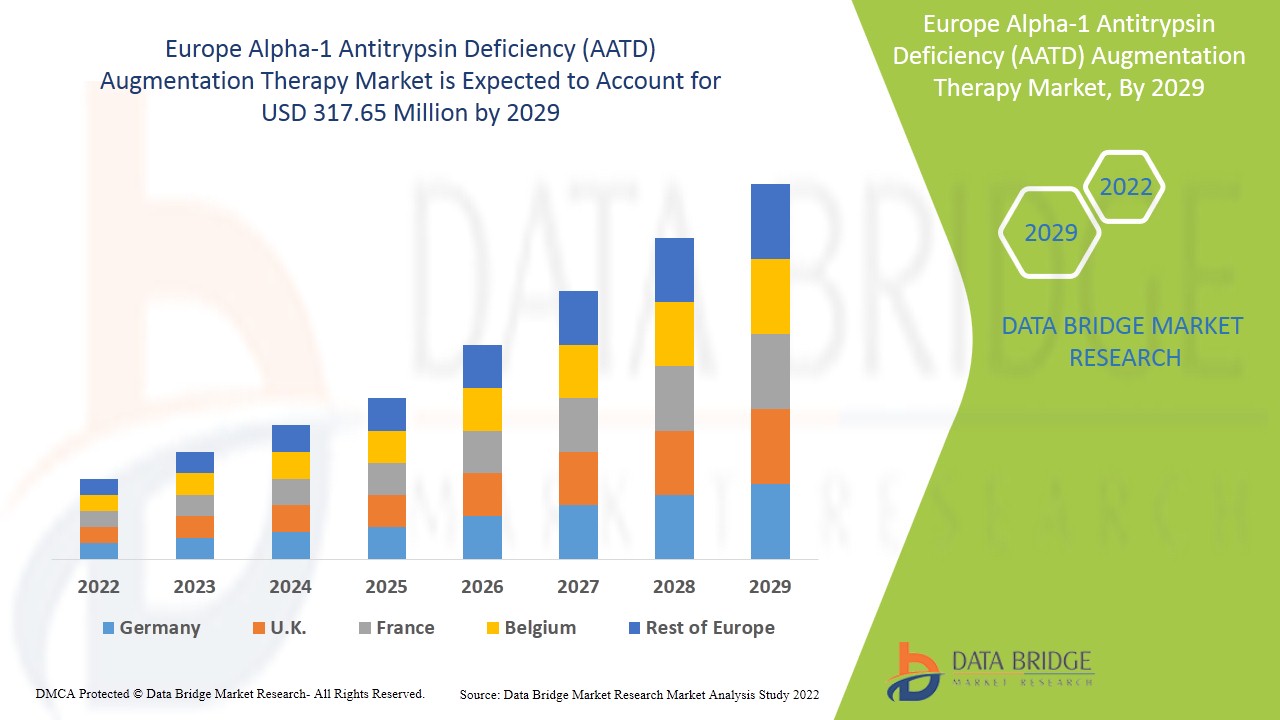
Europe Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market Analysis and Insights
Alpha-1 antitrypsin deficiency (AATD) is the condition that occurs when the liver fails to secrete a protein called alpha-1 antitrypsin that must be released into the blood-stream for various functions such as protecting tissues in the body from being attacked by body’s secreted enzymes. This genetic condition can progress to lung or liver diseases if left untreated. Most patients with this deficiency will either have less protein or do not produce the protein at all and therefore lose its function, leading to abnormal protein produced within the liver cells, causing liver injury.
Augmentation therapy is the only specific treatment licensed for patients with AATD associated lung disease. The therapy uses alpha-1 antitrypsin protein derived from the blood of healthy donors to increase the amount of the protein in the lungs of AAT deficiency patients. This therapy is also called replacement therapy, though it can’t reverse the lung damage that has already occurred but can slow down the further deterioration of lung function. The protein is usually given through an intravenous route with needle insertion.
Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is expected to reach USD 317.65 million by 2029 from USD 179.24 million in 2021, growing with a CAGR of 8.3% in the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Price in USD |
|
Segments Covered |
By Drugs (Prolastin, Aralast NP, Zemaira/Respreeza, Glassia And Others), Gene Type (Type Pimz, Type Pims, Type Pizz And Others), Application (Lung Disease And Liver Disease), Population Type (Adults And Pediatric), End User (Hospitals, Specialty Clinics, Home Healthcare, And Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others) |
|
Countries Covered |
Germany, U.K., France, Italy, Spain, Netherlands, Switzerland, Russia, Turkey and Rest of Europe |
|
Market Players Covered |
Grifols, S.A. (Spain), CSL, Kamada Pharmaceuticals., Takeda Pharmaceutical Company Limited., and LFB BIOMEDICAMENTS. The Pipeline companies which are dealing in market includes Arrowhead Pharmaceuticals, Inc., Mereo BioPharma Group plc, Inhibrx, Inc., Centessa Pharmaceuticals (Z Factor)., Intellia Therapeutics, Inc., Apic Bio, Krystal Biotech, Beam Therapeutics, and LOGICBIO THERAPEUTICS, INC., among others |
Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market Dynamics
Drivers
- Rise in Incidence and Prevalence of AATD
With the increase prevalence and incidence of AATD worldwide, there is an increase in the demand for effective therapy products which will increase in upcoming years. Therefore, rising incidences are expected to act as a driver for the growth of the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market.
- Wide Range of Risks And Comorbidities with AATD
Another significant factor influencing the growth rate of alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is the wide range of risks and comorbidities with AATD. External factors such ach smoking and alcohol consumption also affect severe outcomes directly. Progressive risk to live and lung health will aid the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market.
- Improving Diagnostic Techniques for Genetic Disorders
Growing prevalence of alpha-1 antitrypsin deficiency (AATD) in various regions worldwide and various infections associated with it are expected to act as drivers for development in rare disease diagnostics. Accurate and reliable diagnosis can lead to better quality of life for patients and hence the growth of alpha-1 antitrypsin deficiency (AATD) augmentation therapy market.
Furthermore, improved technology for production and purification methods of AAT, rising initiatives by public and private organizations to spread awareness and growing government funding are the factors that will expand the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market.

Opportunities
- Growing R & D Activities
The Europe food and drug has approved several products currently available in the United States to treat emphysema in patients with AATD. However complete cure for alpha-1 antitrypsin deficiency (AATD) does not exist and hence gives way for new research and opportunity for development of better more effective therapies.
Also, the strategic initiatives by key players and rise in healthcare expenditure will provide structural integrity and future opportunities for the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market in the forecast period of 2022-2029.
Restraints/Challenges
However, high cost of therapies and side effects and complications of augmentation therapies will impede the growth rate of alpha-1 antitrypsin deficiency (AATD) augmentation therapy market. Additionally, difficulty in specific identification and stringent rules and regulations will further challenge the market in the forecast period mentioned above.
This alpha-1 antitrypsin deficiency (AATD) augmentation therapy market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on alpha-1 antitrypsin deficiency (AATD) augmentation therapy market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post COVID-19 Impact on Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market
The COVID-19 has negatively affected the market. Lockdowns and isolation during pandemics complicate the disease management and medication adherence. The lack of access to health-care facilities for routine treatment and medication administration will further affect the market. Social isolation increases stress, despair, and social support, all of which may cause a reduction in sepsis medication adherence during the pandemic.
Recent Development
- In October, 2020, Takeda Pharmaceutical Company Limited announced the collaboration and licensing agreement with Arrowhead Pharmaceuticals Inc. to develop ARO-AAT, a Phase 2 investigational RNA interference (RNAi) therapy in development to treat alpha-1 antitrypsin-associated liver disease (AATLD).
The Europe Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market Scope
The Europe Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy market is segmented into techniques and test type. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Drugs
- Prolastin
- Aralast NP
- Zemaira/Respreeza
- Glassia
- Others
On the basis of drugs, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into prolastin, aralast NP, zemaira/respreeza, glassia and others.
Gene Type
- Type PIMZ
- Type PIMS
- Type PIZZ
- Others
On the basis of gene type, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into type PiMZ, type PiMS, type PiZZ and others.
Application
- Lung Disease
- Liver Disease
On the basis of application, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into lung disease and liver disease.
Population Type
- Adults
- Pediatric
On the basis of population type, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into adults and pediatric.
End User
- Hospitals
- Specialty Clinics
- Home Healthcare
- Others
On the basis of end user, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into hospitals, specialty clinics, home healthcare, and others.
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
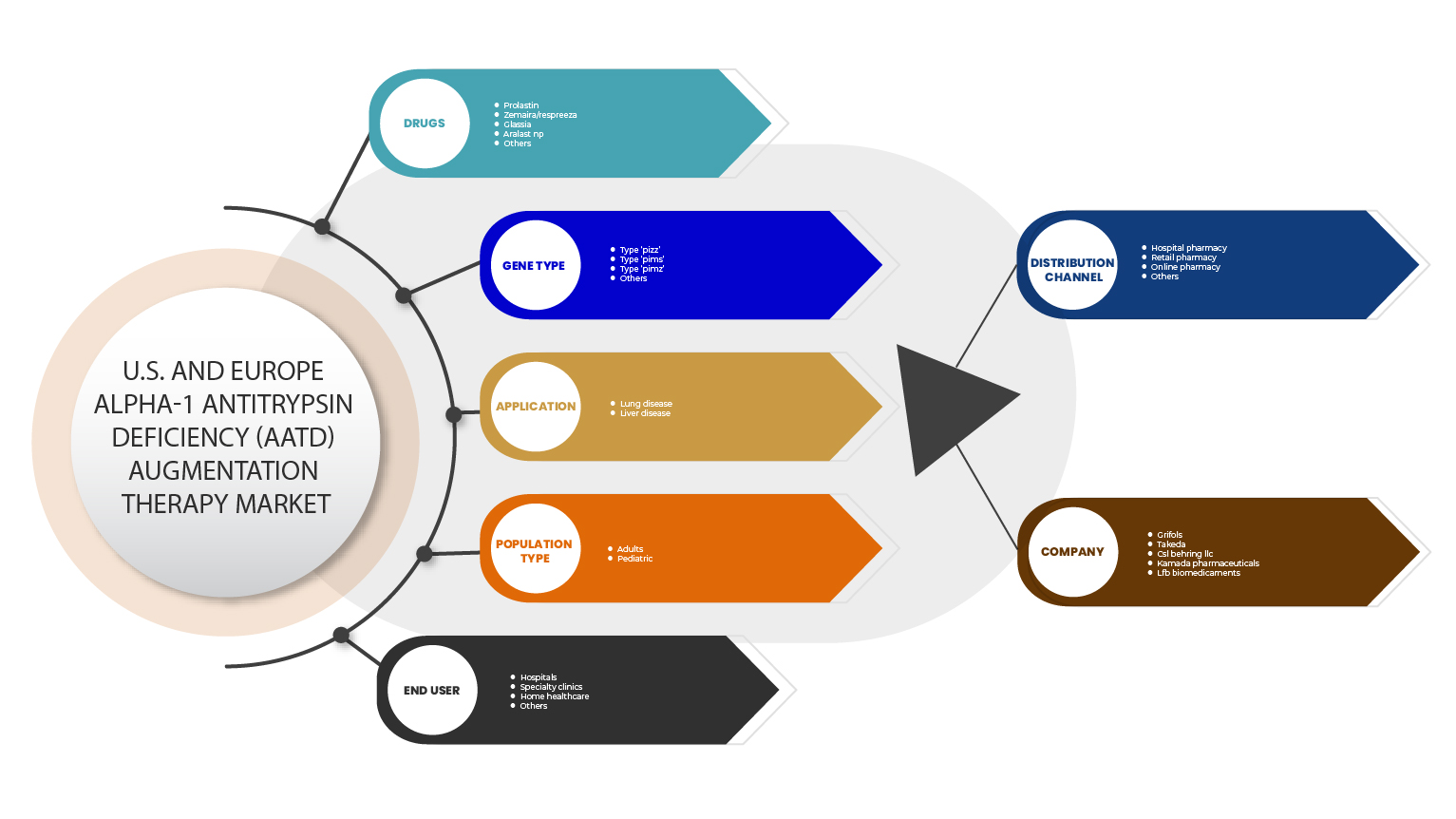
On the basis of distribution channel, the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is segmented into hospital pharmacy, retail pharmacy, online pharmacy and others.
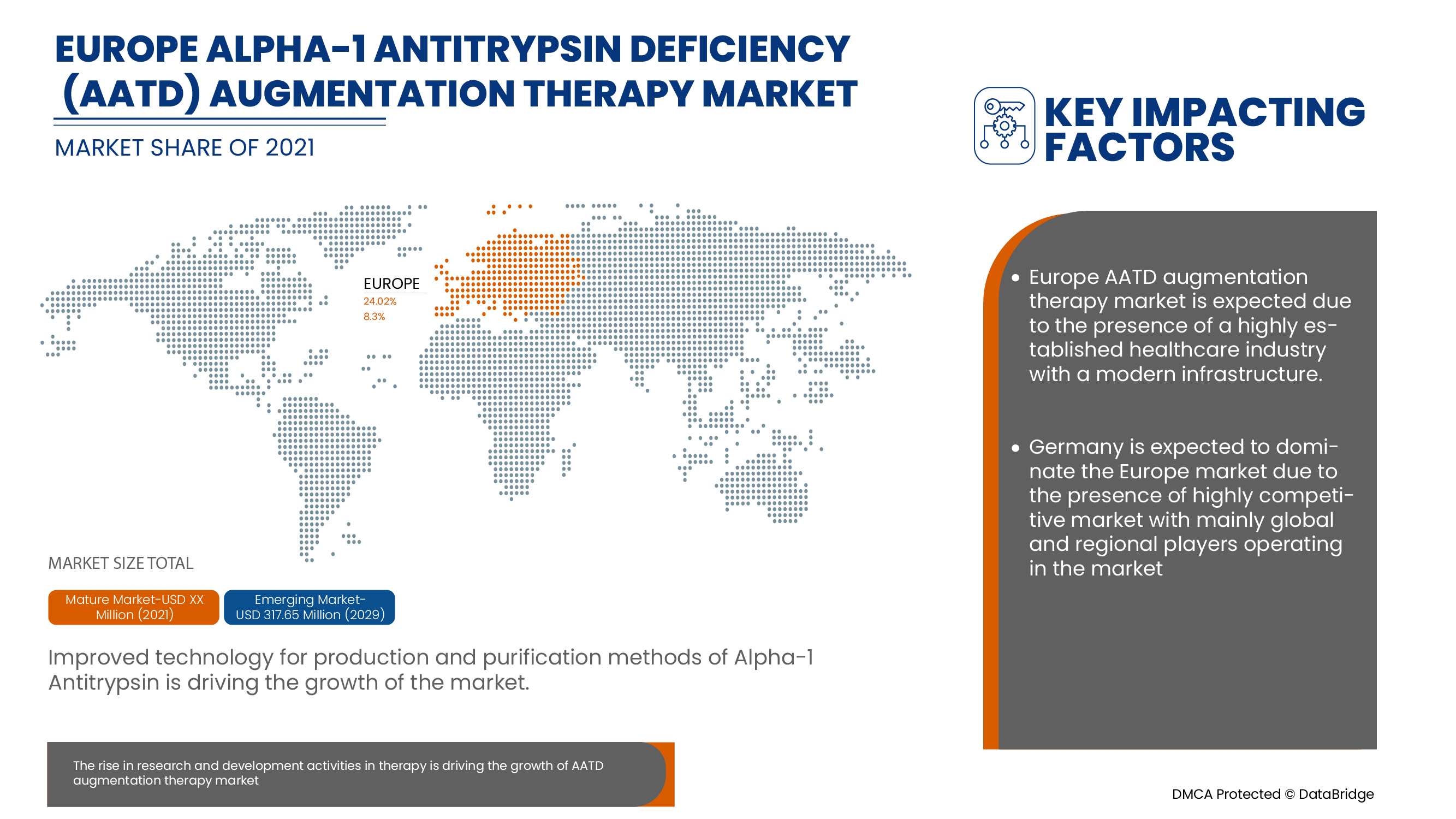
Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market Regional Analysis/Insights
The alpha-1 antitrypsin deficiency (AATD) augmentation therapy market is analysed and market size insights and trends are provided by country, drugs, gene type, population type, application, end user and distribution channel as referenced above.
The countries covered in the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market report are Germany, U.K., France, Italy, Spain, Netherlands, Switzerland, Russia, Turkey and Rest of Europe.
Germany dominates the Europe alpha-1 antitrypsin deficiency (AATD) augmentation therapy market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the increasing healthcare expenditure in Germany, remaining at the highest level among European Union member states, is further improving the country’s market growth.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market Share Analysis
The alpha-1 antitrypsin deficiency (AATD) augmentation therapy market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on alpha-1 antitrypsin deficiency (AATD) augmentation therapy market.
Some of the major players operating in the alpha-1 antitrypsin deficiency (AATD) augmentation therapy market are Grifols, S.A. (Spain), CSL, Kamada Pharmaceuticals., Takeda Pharmaceutical Company Limited., and LFB BIOMEDICAMENTS. The Pipeline companies which are dealing in market includes Arrowhead Pharmaceuticals, Inc., Mereo BioPharma Group plc, Inhibrx, Inc., Centessa Pharmaceuticals (Z Factor)., Intellia Therapeutics, Inc., Apic Bio, Krystal Biotech, Beam Therapeutics, and LOGICBIO THERAPEUTICS, INC., among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analysed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Global Vs Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 DRUGS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 EPIDEMIOLOGY
4.2 PESTEL
4.3 PORTER'S FIVE
4.4 PIPELINE ANALYSIS
5 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: REGULATIONS
5.1 REGULATION IN U.S.:
5.2 REGULATION IN EUROPE:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISE IN INCIDENCE AND PREVALENCE OF AATD
6.1.2 WIDE RANGE OF RISKS AND COMORBIDITIES WITH AATD
6.1.3 IMPROVING DIAGNOSTIC TECHNIQUES FOR GENETIC DISORDERS
6.1.4 IMPROVED TECHNOLOGY FOR PRODUCTION AND PURIFICATION METHODS OF AAT
6.2 RESTRAINTS
6.2.1 HIGH COST OF THERAPIES
6.2.2 SIDE EFFECTS AND COMPLICATIONS OF AUGMENTATION THERAPIES
6.3 OPPORTUNITIES
6.3.1 GROWING R & D ACTIVITIES
6.3.2 STRATEGIC INITIATIVES BY KEY PLAYERS
6.3.3 RISE IN HEALTHCARE EXPENDITURE
6.4 CHALLENGES
6.4.1 DIFFICULTY IN SPECIFIC IDENTIFICATION OF AATD
6.4.2 STRINGENT RULES AND REGULATIONS
7 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS
7.1 OVERVIEW
7.2 PROLASTIN
7.3 ZEMAIRA/RESPREEZA
7.4 GLASSIA
7.5 ARALAST NP
7.6 OTHERS
8 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE
8.1 OVERVIEW
8.2 TYPE ‘PIZZ’
8.3 TYPE ‘PIMS’
8.4 TYPE ‘PIMZ’
8.5 OTHERS
9 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 LUNG DISEASE
9.2.1 EMPHYSEMA
9.2.2 COPD
9.2.3 LUNG CANCER
9.3 LIVER DISEASE
9.3.1 CIRRHOSIS
9.3.2 HEPATITIS
9.3.3 LIVER FAILURE
10 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE
10.1 OVERVIEW
10.2 ADULTS
10.3 PEDIATRIC
11 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 SPECIALTY CLINICS
11.4 HOME HEALTHCARE
11.5 OTHERS
12 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACY
12.3 RETAIL PHARMACY
12.4 ONLINE PHARMACY
12.5 OTHERS
13 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 U.K.
13.1.3 FRANCE
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 NETHERLANDS
13.1.7 SWITZERLAND
13.1.8 RUSSIA
13.1.9 TURKEY
13.1.10 REST OF EUROPE
14 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 GRIFOLS, S.A.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENTS
16.2 CSL
16.2.1 COMPANY SNAPSHOT
16.2.2 PRODUCT PORTFOLIO
16.2.3 RECENT DEVELOPMENT
16.3 TAKEDA PHARMACEUTICAL COMPANY LIMITED
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 KAMADA PHARMACEUTICALS
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 LFB BIOMEDICAMENTS
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT DEVELOPMENT
16.6 APIC BIO
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENT
16.7 ARROWHEAD PHARMACEUTICALS, INC.
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 BEAM THERAPEUTICS
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 CENTESSA PHARMACEUTICALS (Z FACTOR)
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 INHIBRX, INC.
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 INTELLIA THERAPEUTICS, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 KRYSTAL BIOTECH
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 LOGICBIO THERAPEUTICS, INC.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 MEREO BIOPHARMA GROUP PLC
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, PIPELINE ANALYSIS
TABLE 2 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 3 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 4 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 5 U.S. LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 6 U.S. LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 9 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 10 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 11 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 12 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 13 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 17 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 19 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 20 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 21 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 22 GERMANY LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 23 GERMANY LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 24 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 25 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 26 GERMANY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 27 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 28 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 29 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 30 U.K. LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 31 U.K. LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 33 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 U.K. ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 35 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 36 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 37 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 38 FRANCE LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 39 FRANCE LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 40 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 41 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 FRANCE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 43 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 44 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 45 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 46 ITALY LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 47 ITALY LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 48 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 49 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 ITALY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 51 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 52 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 53 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 54 SPAIN LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 55 SPAIN LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 56 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 57 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 58 SPAIN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 59 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 60 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 61 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 62 NETHERLANDS LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 NETHERLANDS LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 64 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 65 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 66 NETHERLANDS ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 67 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 68 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 69 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 70 SWITZERLAND LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 71 SWITZERLAND LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 72 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 73 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 SWITZERLAND ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 76 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 77 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 78 RUSSIA LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 79 RUSSIA LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 80 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 81 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 82 RUSSIA ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 83 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
TABLE 84 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 85 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 86 TURKEY LUNG DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 87 TURKEY LIVER DISEASE IN ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY POPULATION TYPE, 2020-2029 (USD MILLION)
TABLE 89 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 90 TURKEY ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 91 REST OF EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET, BY DRUGS, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: SEGMENTATION
FIGURE 2 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: DROC ANALYSIS
FIGURE 4 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: REGIONAL VS COUNTRY MARKET ANALYSIS
FIGURE 5 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: SEGMENTATION
FIGURE 11 RISING PREVALENCE AND AWARENESS OF AATD IS EXPECTED TO DRIVE THE EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 PROLASTIN SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET
FIGURE 14 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DRUGS, 2021
FIGURE 15 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DRUGS, 2022-2029 (USD MILLION)
FIGURE 16 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DRUGS, CAGR (2022-2029)
FIGURE 17 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 18 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY GENE TYPE, 2021
FIGURE 19 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY GENE TYPE, 2022-2029 (USD MILLION)
FIGURE 20 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY GENE TYPE, CAGR (2022-2029)
FIGURE 21 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY GENE TYPE, LIFELINE CURVE
FIGURE 22 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY APPLICATION, 2021
FIGURE 23 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 24 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 25 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 26 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY POPULATION TYPE, 2021
FIGURE 27 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY AGE GROUP, 2022-2029 (USD MILLION)
FIGURE 28 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY POPULATION TYPE, CAGR (2022-2029)
FIGURE 29 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 30 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY END USER, 2021
FIGURE 31 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 32 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 33 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 35 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 36 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 37 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: SNAPSHOT (2021)
FIGURE 39 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY COUNTRY (2021)
FIGURE 40 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: BY DRUGS (2022-2029)
FIGURE 43 EUROPE ALPHA-1 ANTITRYPSIN DEFICIENCY (AATD) AUGMENTATION THERAPY MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.