1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
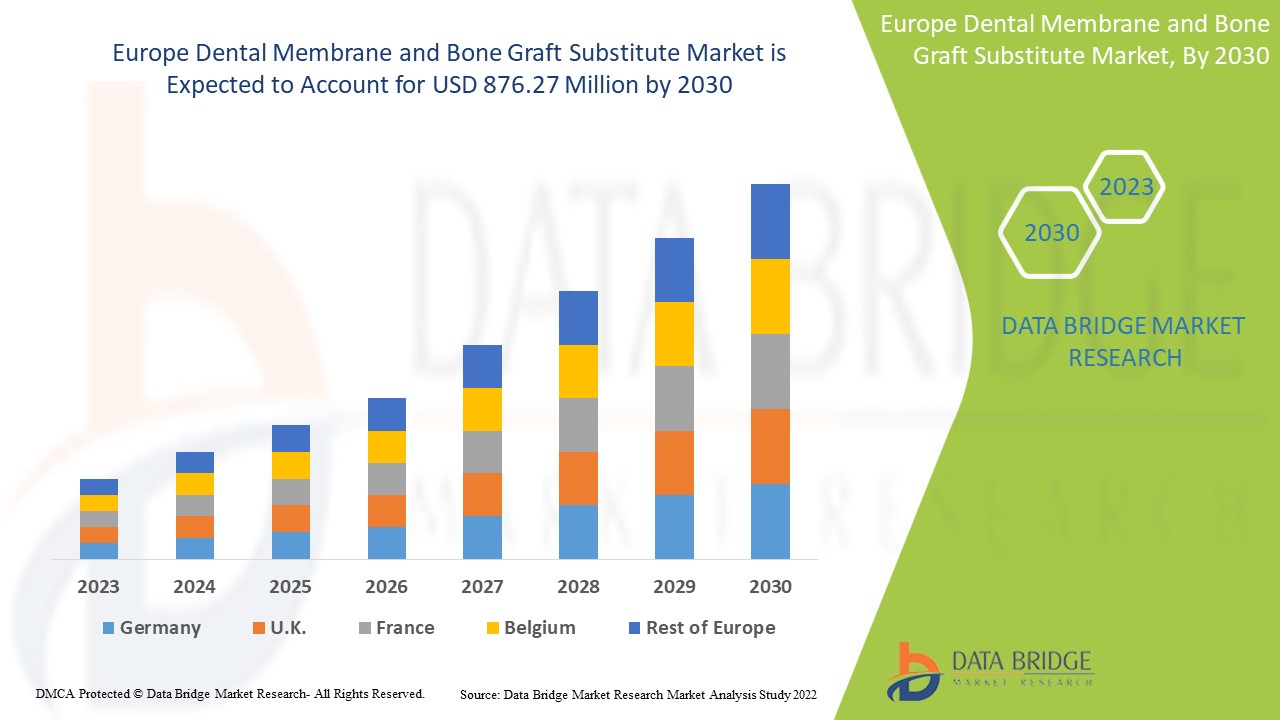
1.3 OVERVIEW OF EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY TYPE
17.1 OVERVIEW
17.2 DENTAL MEMBRANE
17.2.1 SYNTHETIC
17.2.1.1. METAL AND METAL REINFORCED MEMBRANES
17.2.1.2. BILAYER POLYLACTIC ACID (PLA) MEMBRANE
17.2.1.3. PTFE MEMBRANE
17.2.1.4. OTHERS
17.2.2 NATRUAL
17.2.2.1. COLLAGEN MEMBRANE
17.2.2.2. ALLOGRAFT DENTAL MEMBRANES
17.2.2.3. OTHERS
17.3 DENTAL BONE GRAFT SUBSITUTE
17.3.1 SYNTHETIC BONE GRAFT
17.3.1.1. TRICALCIUM PHOSPHATE CERAMICS
17.3.1.2. HYDROXYAPATITE
17.3.1.3. BIOACTIVE GLASS
17.3.1.4. BIPHASIC CALCIUM PHOSPHATE CERAMICS
17.3.1.5. CALCIUM PHOSPHATE CEMENT
17.3.1.6. CALCIUM-PHOSPHOSILICATE
17.3.1.7. METALS
17.3.1.7.1. MAGNESIUM (MG)
17.3.1.7.2. STRONTIUM (SR)
17.3.1.7.3. ZINC (ZN)
17.3.1.7.4. SILICON (SI)
17.3.1.8. POLYMERS
17.3.1.8.1. BY USABILITY
17.3.1.8.1.1 DEGRADABLE
17.3.1.8.1.2 NON-DEGRADABLE
17.3.1.8.2. BY TYPE
17.3.1.8.2.1 POLYLACTIC ACID
17.3.1.8.2.2 POLYGLYCOLIC ACID
17.3.1.8.2.3 POLYΕ-CAPROLACTONE
17.3.1.8.2.4 OTHERS
17.3.1.9. COMPOSITES
17.3.1.9.1. BY TYPE
17.3.1.9.1.1 NANOCRYSTALLINE HA/SILICON DIOXIDE
17.3.1.9.1.2 Β-TCP/CALCIUM SULPHATE
17.3.2 NATURAL BONE GRAFT
17.3.2.1. XENOGRAFT
17.3.2.1.1. BOVINE-DERIVED
17.3.2.1.2. PORCINE-DERIVED
17.3.2.1.3. CHITOSAN DERIVED
17.3.2.1.4. OTHERS
17.3.2.2. ALLOGRAFT
17.3.2.2.1. FRESH OR FRESH-FROZEN BONE
17.3.2.2.1.1 BY TYPE
17.3.2.2.1.1.1. DEMINERALISED BONE ALLOGRAFT
17.3.2.2.1.1.2. OCELLULAR BONE ALLOGRAFTS
17.3.2.2.1.1.3. MACHINED ALLOGRAFTS
17.3.2.2.1.2 BY USABLITY
17.3.2.2.1.2.1. BIO-REABSORBABLE
17.3.2.2.1.2.2. NON-BIO-REABSORBABLE
17.3.2.2.2. FREEZE DRIED BONE ALLOGRAFT (FDBA)
17.3.2.2.2.1 BY TYPE
17.3.2.2.2.1.1. DEMINERALISED BONE ALLOGRAFT
17.3.2.2.2.1.2. OCELLULAR BONE ALLOGRAFTS
17.3.2.2.2.1.3. MACHINED ALLOGRAFTS
17.3.2.2.2.2 BY USABILITY
17.3.2.2.2.2.1. BIO-REABSORBABLE
17.3.2.2.2.2.2. NON-BIO-REABSORBABLE
17.3.2.3. AUTOGRAFTS
17.3.2.3.1. MANDIBULAR SYMPHYSIS
17.3.2.3.2. MANDIBULAR RAMUS
17.3.2.3.3. EXTERNAL OBLIQUE RIDGE
17.3.2.3.4. ILIAC CREST
17.3.2.3.5. PROXIMAL ULNA
17.3.2.3.6. DISTAL RADIUS
17.3.2.4. PHYTOGENIC MATERIALS
17.3.2.4.1. GUSUIBU
17.3.2.4.2. CORAL-BASED BONE SUBSTITUTES
17.3.2.4.3. MARINE ALGAE
17.3.3 LIVE OSTEOGENIC CELLS BONE SUBSTITUTES
17.3.3.1. BIOSEED-ORAL BONE
17.3.3.2. OSTEOTRANSPLANT DENT
17.3.4 GROWTH FACTORS
17.3.4.1. TRANSFORMING GROWTH FACTOR-BETA (TGF-BETA)
17.3.4.2. PLATELET-DERIVED GROWTH FACTOR (PDGF)
17.3.4.3. FIBROBLAST GROWTH FACTORS (FGF)
17.3.4.4. BONE MORPHOGENEIC PROTEIN (BMP)
17.3.4.5. OTHERS
18 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY APPLICATION
18.1 OVERVIEW
18.2 RIDGE AUGMENTATION
18.2.1 BY TYPE
18.2.1.1. DENTAL MEMBRANE
18.2.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.2.2 BY SOURCE
18.2.2.1. NATURAL
18.2.2.2. SYNTHETIC
18.3 SOCKET PRESERVATION
18.3.1 BY TYPE
18.3.1.1. DENTAL MEMBRANE
18.3.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.3.2 BY SOURCE
18.3.2.1. NATURAL
18.3.2.2. SYNTHETIC
18.4 PERIODONTAL DEFECT REGENERATION
18.4.1 BY TYPE
18.4.1.1. DENTAL MEMBRANE
18.4.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.4.2 BY SOURCE
18.4.2.1. NATURAL
18.4.2.2. SYNTHETIC
18.5 IMPLANT BONE REGENERATION
18.5.1 BY TYPE
18.5.1.1. DENTAL MEMBRANE
18.5.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.5.2 BY SOURCE
18.5.2.1. NATURAL
18.5.2.2. SYNTHETIC
18.6 SINUS LIFT
18.6.1 BY TYPE
18.6.1.1. DENTAL MEMBRANE
18.6.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.6.2 BY SOURCE
18.6.2.1. NATURAL
18.6.2.2. SYNTHETIC
18.7 OTHERS
19 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY SOURCE
19.1 OVERVIEW
19.2 NATURAL
19.3 SYNTHETIC
20 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY USABILITY
20.1 OVERVIEW
20.2 RESORBABLE
20.3 NON-RESORBABLE
21 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY MECHANISM
21.1 OVERVIEW
21.2 OSTEOCONDUCTION
21.3 OSTEOINDUCTION
21.4 OSTEOPROMOTION
21.5 OSTEOGENESIS
22 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY AGE
22.1 OVERVIEW
22.2 PEDIATRICS
22.3 ADULT
22.4 GERIATRIC
23 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY POPULATION TYPE
23.1 OVERVIEW
23.2 MALE
23.3 FEMALE
24 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY END USER
24.1 OVERVIEW
24.2 HOSPITALS
24.3 DENTAL CLINICS
24.4 DENTAL LABORATORIES
24.5 AMBULATORY SURGICAL CENTERS
24.6 TRAUMA CENTER
24.7 RESEARCH AND DENTAL LABORATORIES
24.8 OTHERS
25 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY DISTRIBUTION CHANNEL
25.1 OVERVIEW
25.2 DIRECT TENDER
25.3 RETAIL SALES
25.3.1 HOSPITAL PHARMACY
25.3.2 RETAIL PHARMACY
25.3.3 ONLINE PHARMACY
25.4 OTHERS
26 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, SWOT AND DBMR ANALYSIS
27 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, COMPANY LANDSCAPE
27.1 COMPANY SHARE ANALYSIS: EUROPE
27.2 MERGERS & ACQUISITIONS
27.3 NEW PRODUCT DEVELOPMENT & APPROVALS
27.4 EXPANSIONS
27.5 REGULATORY CHANGES
27.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
28 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY REGION
EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
OVERVIEW (ALL SEGMENTATION PROVIDED ABOVE IS REPRESNTED IN THIS CHAPTER BY COUNTRY)
28.1 EUROPE
28.1.1 GERMANY
28.1.2 U.K.
28.1.3 ITALY
28.1.4 FRANCE
28.1.5 SPAIN
28.1.6 SWITZERLAND
28.1.7 NETHERLANDS
28.1.8 BELGIUM
28.1.9 RUSSIA
28.1.10 TURKEY
28.1.11 DENMARK
28.1.12 NORWAY
28.1.13 POLAND
28.1.14 SWEDEN
28.1.15 GREECE
28.1.16 REST OF EUROPE
28.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
29 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, COMPANY PROFILE
29.1 ZIMMER BIOMET
29.1.1 COMPANY OVERVIEW
29.1.2 REVENUE ANALYSIS
29.1.3 GEOGRAPHIC PRESENCE
29.1.4 PRODUCT PORTFOLIO
29.1.5 RECENT DEVELOPMENTS
29.2 BIOVENTUS
29.2.1 COMPANY OVERVIEW
29.2.2 REVENUE ANALYSIS
29.2.3 GEOGRAPHIC PRESENCE
29.2.4 PRODUCT PORTFOLIO
29.2.5 RECENT DEVELOPMENTS
29.3 BIOHORIZONS ( HENRY SCHEIN, INC.)
29.3.1 COMPANY OVERVIEW
29.3.2 REVENUE ANALYSIS
29.3.3 GEOGRAPHIC PRESENCE
29.3.4 PRODUCT PORTFOLIO
29.3.5 RECENT DEVELOPMENTS
29.4 DENTSPLY SIRONA
29.4.1 COMPANY OVERVIEW
29.4.2 REVENUE ANALYSIS
29.4.3 GEOGRAPHIC PRESENCE
29.4.4 PRODUCT PORTFOLIO
29.4.5 RECENT DEVELOPMENTS
29.5 ZIMVIE INC.
29.5.1 COMPANY OVERVIEW
29.5.2 REVENUE ANALYSIS
29.5.3 GEOGRAPHIC PRESENCE
29.5.4 PRODUCT PORTFOLIO
29.5.5 RECENT DEVELOPMENTS
29.6 OSTEOGENICS BIOMEDICAL
29.6.1 COMPANY OVERVIEW
29.6.2 REVENUE ANALYSIS
29.6.3 GEOGRAPHIC PRESENCE
29.6.4 PRODUCT PORTFOLIO
29.6.5 RECENT DEVELOPMENTS
29.7 GEISTLICH PHARMA AG
29.7.1 COMPANY OVERVIEW
29.7.2 REVENUE ANALYSIS
29.7.3 GEOGRAPHIC PRESENCE
29.7.4 PRODUCT PORTFOLIO
29.7.5 RECENT DEVELOPMENTS
29.8 MEDTRONIC
29.8.1 COMPANY OVERVIEW
29.8.2 REVENUE ANALYSIS
29.8.3 GEOGRAPHIC PRESENCE
29.8.4 PRODUCT PORTFOLIO
29.8.5 RECENT DEVELOPMENTS
29.9 INSTITUT STRAUMANN AG
29.9.1 COMPANY OVERVIEW
29.9.2 REVENUE ANALYSIS
29.9.3 GEOGRAPHIC PRESENCE
29.9.4 PRODUCT PORTFOLIO
29.9.5 RECENT DEVELOPMENTS
29.1 BOTISS BIOMATERIALS GMBH
29.10.1 COMPANY OVERVIEW
29.10.2 REVENUE ANALYSIS
29.10.3 GEOGRAPHIC PRESENCE
29.10.4 PRODUCT PORTFOLIO
29.10.5 RECENT DEVELOPMENTS
29.11 DENTIUMUSA
29.11.1 COMPANY OVERVIEW
29.11.2 REVENUE ANALYSIS
29.11.3 GEOGRAPHIC PRESENCE
29.11.4 PRODUCT PORTFOLIO
29.11.5 RECENT DEVELOPMENTS
29.12 NOVABONE PRODUCTS, LLC. A HALMA COMPANY
29.12.1 COMPANY OVERVIEW
29.12.2 REVENUE ANALYSIS
29.12.3 GEOGRAPHIC PRESENCE
29.12.4 PRODUCT PORTFOLIO
29.12.5 RECENT DEVELOPMENTS
29.13 CGBIO
29.13.1 COMPANY OVERVIEW
29.13.2 REVENUE ANALYSIS
29.13.3 GEOGRAPHIC PRESENCE
29.13.4 PRODUCT PORTFOLIO
29.13.5 RECENT DEVELOPMENTS
29.14 REGENITY
29.14.1 COMPANY OVERVIEW
29.14.2 REVENUE ANALYSIS
29.14.3 GEOGRAPHIC PRESENCE
29.14.4 PRODUCT PORTFOLIO
29.14.5 RECENT DEVELOPMENTS
29.15 NEOSS
29.15.1 COMPANY OVERVIEW
29.15.2 REVENUE ANALYSIS
29.15.3 GEOGRAPHIC PRESENCE
29.15.4 PRODUCT PORTFOLIO
29.15.5 RECENT DEVELOPMENTS
29.16 BIOMATLANTE
29.16.1 COMPANY OVERVIEW
29.16.2 REVENUE ANALYSIS
29.16.3 GEOGRAPHIC PRESENCE
29.16.4 PRODUCT PORTFOLIO
29.16.5 RECENT DEVELOPMENTS
29.17 GRAFTYS
29.17.1 COMPANY OVERVIEW
29.17.2 REVENUE ANALYSIS
29.17.3 GEOGRAPHIC PRESENCE
29.17.4 PRODUCT PORTFOLIO
29.17.5 RECENT DEVELOPMENTS
29.18 DEPUY SYNTHES (JOHNSON & JOHNSON)
29.18.1 COMPANY OVERVIEW
29.18.2 REVENUE ANALYSIS
29.18.3 GEOGRAPHIC PRESENCE
29.18.4 PRODUCT PORTFOLIO
29.18.5 RECENT DEVELOPMENTS
29.19 BONESUPPORT AB
29.19.1 COMPANY OVERVIEW
29.19.2 REVENUE ANALYSIS
29.19.3 GEOGRAPHIC PRESENCE
29.19.4 PRODUCT PORTFOLIO
29.19.5 RECENT DEVELOPMENTS
29.2 WRIGHT MEDICAL GROUP N.V.
29.20.1 COMPANY OVERVIEW
29.20.2 REVENUE ANALYSIS
29.20.3 GEOGRAPHIC PRESENCE
29.20.4 PRODUCT PORTFOLIO
29.20.5 RECENT DEVELOPMENTS
29.21 CURASAN AG
29.21.1 COMPANY OVERVIEW
29.21.2 REVENUE ANALYSIS
29.21.3 GEOGRAPHIC PRESENCE
29.21.4 PRODUCT PORTFOLIO
29.21.5 RECENT DEVELOPMENTS
30 RELATED REPORTS
31 CONCLUSION
32 QUESTIONNAIRE
33 ABOUT DATA BRIDGE MARKET RESEARCH