Global Cardiac Marker Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
13.83 Billion
USD
27.76 Billion
2024
2032
USD
13.83 Billion
USD
27.76 Billion
2024
2032
| 2025 –2032 | |
| USD 13.83 Billion | |
| USD 27.76 Billion | |
|
|
|
|
Cardiac Marker Testing Market Size
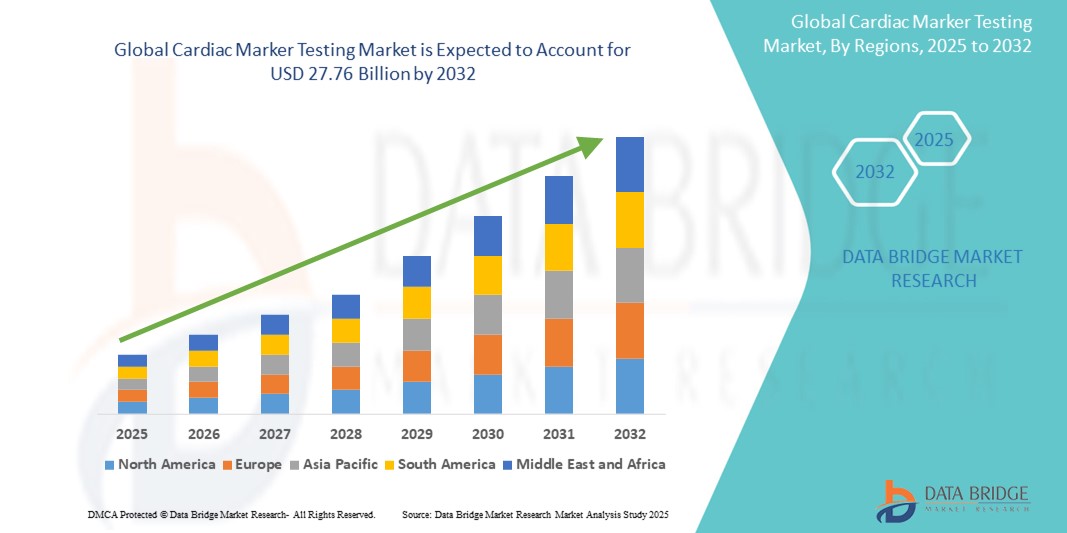
- The global cardiac marker testing market size was valued at USD 13.83 billion in 2024 and is expected to reach USD 27.76 billion by 2032, at a CAGR of 9.10% during the forecast period
- The market growth for cardiac marker testing is largely fueled by the increasing prevalence of cardiovascular diseases (CVDs) such as myocardial infarction, acute coronary syndrome, and congestive heart failure, which require accurate and timely diagnosis
- Furthermore, rising demand for user-friendly drug delivery systems, coupled with the need for effective and safe medication administration during critical care and emergencies, is establishing cardiac marker testing as essential tools for both patients and healthcare professionals. The shift towards personalized medicine and biomarker-guided therapies, along with increasing healthcare spending and improved infrastructure, are also contributing factors
Cardiac Marker Testing Market Analysis
- Cardiac marker testing involves measuring levels of specific proteins in the blood that are released when the heart muscle is damaged. These tests help diagnose or rule out conditions such as heart attacks and monitor heart health
- The cardiac marker testing market is experiencing significant growth, driven by the increasing prevalence of chronic diseases requiring frequent injections, the rising number of biologics and biosimilars available in pre-filled syringes, and the growing need for user-friendly drug delivery in emergency situations
- North America dominates the cardiac marker testing market with the largest revenue share of 41.2% in 2024 which is attributed to its advanced healthcare infrastructure, high adoption rates of sophisticated medical devices, and a strong presence of leading pharmaceutical and medical device manufacturers
- Asia-Pacific is expected to be the fastest growing region in the cardiac marker testing market during the forecast period, fueled by region's growing inclination towards self-care and advanced diagnostic delivery, supported by government initiatives promoting healthcare modernization
- Reagents and kits segment dominates the cardiac marker testing market with a market share of 65.2% in 2024, driven by their critical role in detecting and quantifying cardiac biomarkers across diverse testing platforms
Report Scope and Cardiac Marker Testing Market Segmentation
|
Attributes |
Cardiac Marker Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cardiac Marker Testing Market Trends
“Enhanced Precision and User Experience through AI and Smart Integration in Cardiac Marker Testing”
- A significant and rapidly accelerating trend within the global cardiac marker testing market is the profound integration of artificial intelligence (AI) and the incorporation of smart features designed to elevate the user experience. This convergence of cutting-edge technologies is fundamentally transforming how cardiac biomarkers are analyzed and managed, providing unprecedented levels of convenience and control
- For instance, sophisticated cardiac marker testing devices are increasingly being developed with advanced connectivity options, such as Bluetooth and Wi-Fi. These enable seamless data transmission, allowing patients to track their biomarker levels and healthcare professionals to monitor trends and adherence to treatment plans remotely
- AI integration within cardiac marker testing platforms empowers features such as improved accuracy in biomarker analysis, the potential for personalized diagnostic insights based on individual patient profiles, and the generation of more intelligent alerts derived from comprehensive usage data
- This progressive shift towards more intelligent, intuitive, and interconnected cardiac marker testing systems is fundamentally reshaping expectations for both in-hospital and home healthcare settings. Consequently, leading companies are actively developing AI-enabled solutions that offer features such as automatic adjustments for precise measurement, real-time data tracking, and highly user-friendly interfaces
- The demand for cardiac marker testing solutions that offer seamless AI and voice control integration (or similar smart functionalities) is experiencing rapid growth across both hospital and home healthcare sectors. This surge is driven by a strong collective prioritization of convenience, accuracy, and comprehensive smart health functionality by patients and clinicians alike
Cardiac Marker Testing Market Dynamics
Driver
“Growing Demand Due to Rising Cardiovascular Disease Burden and Preference for Accessible Diagnostics”
- The escalating prevalence of cardiovascular diseases (CVDs) across a burgeoning global population, coupled with an increasing demand for convenient and accessible diagnostic interventions, is a significant driving force behind the heightened demand for cardiac marker testing. As CVDs continue to be a leading cause of mortality worldwide, the need for accurate and timely diagnostic tools for conditions such as myocardial infarction, heart failure, and acute coronary syndrome is paramount
- For instance, in October 2023, Mindray announced the launch of new high-sensitive troponin I and NT-proBNP cardiac biomarkers. This advancement aims to improve the diagnosis and management of cardiovascular diseases, driving growth in the cardiac marker testing market
- Furthermore, the growing sophistication of patient-friendly diagnostic platforms, particularly point-of-care (POCT) solutions, requiring precise and easy-to-use administration, alongside the desire for interconnected patient monitoring systems, are making cardiac marker testing an integral component of modern healthcare regimens. These systems offer seamless integration with digital health platforms, allowing for remote monitoring and data sharing
- The convenience of accessible testing for individuals at risk of or suffering from chronic cardiovascular conditions, remote monitoring capabilities, and the ability to manage complex diagnostic regimens through integrated smart systems are key factors propelling the adoption of cardiac marker testing in both hospital and home healthcare sectors. The trend towards improved patient experience and the increasing availability of user-friendly testing options further contributes to market growth
Restraint/Challenge
“Concerns Regarding Test Complexity, Standardization, and High Implementation Costs”
- Concerns surrounding the complexity of some advanced cardiac marker testing platforms and the potential for user error or misinterpretation of results pose a significant challenge to broader market penetration. As these diagnostic systems integrate more features and technologies, they can be susceptible to incorrect usage if not accompanied by clear instructions, standardized protocols, and proper training, raising anxieties among healthcare professionals and patients about the reliability of their diagnostic results
- For instance, anecdotal reports of clinicians struggling with multi-step testing processes, or the variability in results across different assays and laboratories, have made some hesitant to fully embrace the most advanced cardiac marker testing solutions without robust standardization
- In addition, the relatively high initial cost of some advanced cardiac marker testing systems, particularly those incorporating cutting-edge technologies or automated platforms, can be a barrier to adoption for resource-constrained healthcare systems or for patients without adequate insurance coverage
- While prices are gradually decreasing due to technological advancements and increased competition, the perceived premium for advanced cardiac marker testing technology can still hinder widespread adoption, especially for regions or healthcare providers who do not see an immediate need for the most sophisticated features offered
Cardiac Marker Testing Market Scope
The market is segmented on the basis of type, product, disease, and type of testing.
- By Type
On the basis of type, the cardiac marker testing market is segmented into troponin I and T, creatine kinase-MB (CK-MB), brain natriuretic peptide (BNP or NT-proBNP), myoglobin, high-sensitivity C-reactive protein (hs-CRP), and other biomarkers. The troponin I and T segment held the largest market share of 41.7% in 2024, due to their unparalleled accuracy and gold standard status in diagnosing acute myocardial infarction (heart attacks).
The brain natriuretic peptide (BNP or NT-proBNP) segment is anticipated to witness the fastest growth rate, fueled by its crucial role in diagnosing heart failure and risk stratification in cardiovascular conditions.
- By Product
On the basis of product, the Cardiac marker testing market is segmented into instruments chemiluminescence, immunofluorescence, ELISA, Immunochromatography and reagents and kits. The Reagents and Kits segment dominated the largest market revenue share of 65.2% in 2024. Their widespread adoption is attributed to their critical role in detecting and quantifying cardiac biomarkers across multiple testing platforms, ensuring high sensitivity and specificity in diagnostic results.
The reagents and kits segment is expected to register a high revenue CAGR during the forecast period due to growing incidence of cardiovascular diseases, ease of use, and improved test accuracy, which is leading to an increase in demand for cardiac marker tests.
- By Disease
On the basis of disease, the cardiac marker testing market is segmented into myocardial infarction, congestive heart failure, acute coronary syndrome, atherosclerosis, and ischemia. The acute coronary syndrome segment held the largest market revenue share of 2024, due to the widespread utilization of key cardiac biomarkers, especially troponins, for its diagnosis.
The myocardial infarction segment is anticipated to grow at a CAGR % over the forecast period, driven by increased research emphasis on this condition
- By Type of Testing
On the basis of type of testing, the cardiac marker testing market is segmented into laboratory testing, academic institutions, and point-of-care testing. The laboratory testing segment held the largest market revenue share in 2024, due to their comprehensive diagnostic capabilities and established infrastructure for conducting cardiac marker tests, offering reliability and high accuracy.
The point-of-care testing segment is anticipated to grow rapidly due to the increasing adoption of sensitive and user-friendly POC troponin tests and the need for immediate results for timely treatment decisions, especially in emergency settings
Cardiac Marker Testing Market Regional Analysis
- North America dominates the cardiac marker testing market with the largest revenue share of 41.2% in 2024, driven by a growing demand for advanced diagnostic care and a high adoption rate of sophisticated medical technologies
- Consumers and healthcare providers in the region highly value the effectiveness and advanced features for patient safety offered by cardiac marker testing within comprehensive healthcare systems, particularly for the accurate diagnosis and monitoring of cardiac conditions. The high prevalence of cardiovascular diseases, for instance, in the U.S., about 1 in every 5 deaths in 2022 was due to heart disease, underscores the critical need for these tests
- This widespread adoption is further supported by high healthcare expenditure, a technologically advanced medical community, favorable reimbursement policies, and the increasing emphasis on early diagnosis and management of cardiac conditions, establishing Cardiac Marker Testing as a favored diagnostic approach across various healthcare environments
Cardiac Marker Testing Market Regional Analysis
U.S. Cardiac Marker Testing Market Insight
The U.S. cardiac marker testing market captured the largest revenue share of 30.4% in 2024 within North America, fueled by the swift uptake of advanced medical technologies and the expanding trend of patient care. Healthcare and emergency medical services are increasingly prioritizing the enhancement of patient safety and treatment efficacy through intelligent, integrated diagnostic solutions. The growing preference for convenient diagnostic techniques, combined with robust demand for advanced monitoring and portable devices, further propels the cardiac marker testing industry. Moreover, the increasing integration of sophisticated software and connectivity features is significantly contributing to the market's expansion.
Europe Cardiac Marker Testing Market Insight
The European cardiac marker testing market is projected to expand at a substantial CAGR from 2025 to 2032, primarily driven by stringent healthcare regulations and the escalating need for rapid and accurate diagnostics in hospitals and homecare settings. The increase in the aging population, coupled with the demand for user-friendly medical devices for chronic conditions, is fostering the adoption of advanced cardiac marker testing technologies. European healthcare providers are also drawn to the enhanced patient outcomes and safety features these diagnostic devices offer. The region is experiencing significant growth across hospital intensive care units, emergency rooms, and home healthcare applications, with cardiac marker testing being incorporated into both new healthcare facilities and upgrades of existing ones.
U.K. Cardiac Marker Testing Market Insight
The U.K. cardiac marker testing market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the escalating trend of home healthcare practices and a desire for heightened patient safety and treatment effectiveness. In addition, concerns regarding chronic disease management and the need for convenient drug delivery solutions are encouraging both hospitals and homecare providers to choose advanced cardiac marker testing technologies. The U.K.’s embrace of technological advancements in healthcare, alongside its robust healthcare infrastructure, is expected to continue to stimulate market growth
Germany Cardiac Marker Testing Market Insight
The German cardiac marker testing market is expected to expand at a considerable CAGR from 2025 to 2034, fueled by increasing awareness of self-care and the demand for technologically advanced, patient-centric solutions. Germany’s well-developed healthcare infrastructure, combined with its emphasis on innovation and patient safety, promotes the adoption of advanced cardiac marker testing devices, particularly in hospital and specialized care settings. The integration of cardiac marker testing with patient monitoring systems is also becoming increasingly prevalent, with a strong preference for secure, reliable solutions aligning with local healthcare standards
Asia-Pacific Cardiac Marker Testing Market Insight
The Asia-Pacific cardiac marker testing market is poised to grow at the fastest CAGR of 11.9% from 2025 to 2032, driven by increasing healthcare investments, rising disposable incomes, and technological advancements in countries such as China, Japan, and India. The region's growing inclination towards self-care and advanced diagnostic delivery, supported by government initiatives promoting healthcare modernization, is driving the adoption of advanced cardiac marker testing devices. Furthermore, as APAC emerges as a manufacturing hub for medical device components and systems, the affordability and accessibility of certain cardiac marker testing technologies are expanding to a wider healthcare base
Japan Cardiac Marker Testing Market Insight
The Japan cardiac marker testing market is gaining momentum due to the country’s high-tech culture, rapid aging population, and demand for convenient healthcare solutions. The Japanese market places a significant emphasis on patient safety and comfort, and the adoption of advanced cardiac marker testing devices is driven by the increasing number of elderly patients and complex medical cases requiring precise diagnosis. The integration of cardiac marker testing with other medical IoT devices and monitoring systems is fueling growth. Moreover, Japan's aging population is likely to spur demand for easier-to-use, reliable diagnostic solutions in both hospital and homecare sectors.
China Cardiac Marker Testing Market Insight
The China cardiac marker testing market accounted for the largest market revenue share in Asia Pacific in 2024 with a CAGR of 15.9%, attributed to the country's expanding healthcare infrastructure, rapid urbanization, and high rates of technological adoption in the medical sector. China stands as one of the largest markets for medical devices, and advanced cardiac marker testing devices are becoming increasingly popular in hospitals, emergency rooms, and specialized clinics. The push towards self-administration of chronic medications and the availability of affordable medical device options, alongside strong domestic manufacturers, are key factors propelling the market in China.
Cardiac Marker Testing Market Share
The cardiac marker testing industry is primarily led by well-established companies, including:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Siemens Healthineers AG (Germany)
- Danaher Corporation (U.S.)
- BIOMÉRIEUX (France)
- QuidelOrtho Corporation (U.S.)
- Randox Laboratories Ltd (U.K.)
- BG Medicine (U.S.)
- Bhat Bio-tech India Private Limited (India)
- Merck KGaA (Germany)
- PerkinElmer (U.S.)
- QIAGEN (Netherlands)
- Agilent Technologies, Inc. (U.S.)
- Bruker (U.S.)
- Epigenomics AG (Germany)
- MESO SCALE DIAGNOSTICS, LLC. (U.S.)
- EKF Diagnostics Holdings plc. (U.K.)
- Nexus-Dx (U.S.)
- LifeSign LLC. (U.S.)
- DIALAB GmbH (Austria)
- Beckman Coulter, Inc. (U.S.)
Latest Developments in Global Cardiac Marker Testing Market
- In November 2023, Abbott Laboratories received FDA clearance for its i-STAT TBI plasma test, which includes advanced cardiac marker detection capabilities. This expands the use of portable point-of-care devices for rapid cardiac and neurological diagnostics in both hospital and field environments
- In June 2024, Siemens Healthineers expanded its cardiac testing portfolio by introducing the NT-proBNPII (PBNPII) assay on the Atellica Solution platform. This development aims to provide enhanced diagnostic capabilities for heart conditions
- In March 2024, Polymedco obtained 510(k) clearance by the FDA for its speedy troponin assay as a unit of its Pathfast Biomarker Analyser. This FDA-approved biomarker identifies the existence of cardiac troponin I, a critical indicator for myocardial infarction
- In October 2023, Mindray, a global provider of medical devices and solutions, launched two new cardiac biomarkers worldwide: high-sensitivity troponin I (hs-cTnI) and NT-proBNP. These additions enhance Mindray's diverse portfolio for diagnosing and managing cardiovascular diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.