Global Lysosomal Acid Lipase Deficiency Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
803.98 Million
USD
1,893.25 Million
2024
2032
USD
803.98 Million
USD
1,893.25 Million
2024
2032
| 2025 –2032 | |
| USD 803.98 Million | |
| USD 1,893.25 Million | |
|
|
|
|
Lysosomal Acid Lipase Deficiency Treatment Market Size
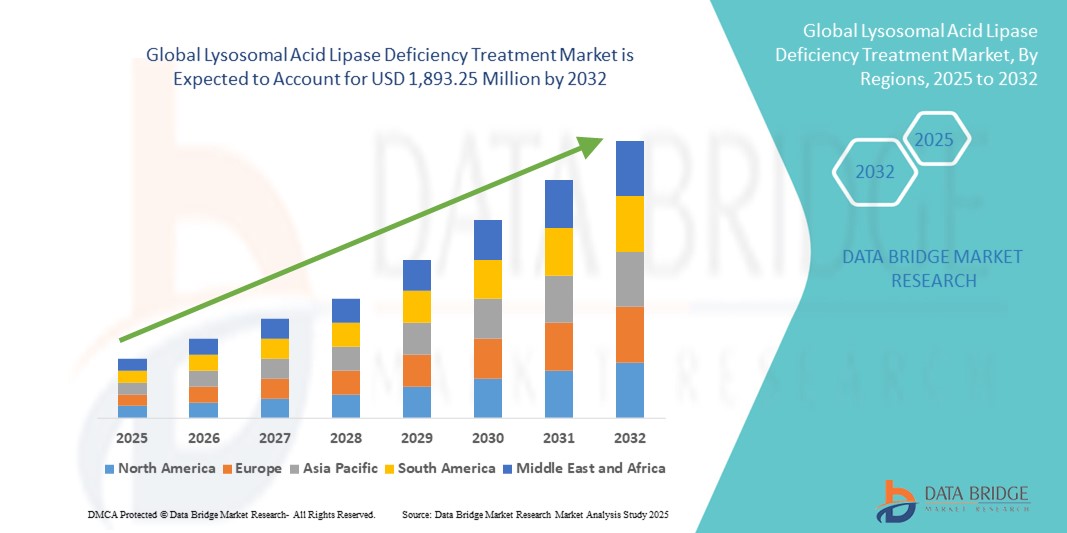
- The global lysosomal acid lipase deficiency treatment market size was valued at USD 803.98 million in 2024 and is expected to reach USD 1,893.25 million by 2032, at a CAGR of 11.30% during the forecast period
- The market growth is primarily driven by increasing awareness of rare genetic disorders, improved diagnostic capabilities, and advancements in enzyme replacement therapies (ERT), particularly for conditions such as Wolman disease and cholesteryl ester storage disease (CESD)
- In addition, rising government initiatives, orphan drug designations, and R&D investments in ultra-rare metabolic conditions are accelerating access to novel therapies, propelling the expansion of this highly specialized treatment landscape
Lysosomal Acid Lipase Deficiency Treatment Market Analysis
- Lysosomal acid lipase (LAL) deficiency treatments, particularly enzyme replacement therapies, are vital in managing this rare lysosomal storage disorder by reducing harmful lipid accumulation in organs and improving survival, especially in early-onset cases such as Wolman disease
- The rising demand for effective LAL deficiency treatments is fueled by increased awareness of rare genetic conditions, advancements in diagnostic screening, and the clinical success of sebelipase alfa as a targeted therapy
- North America dominated the lysosomal acid lipase deficiency treatment market with the largest revenue share of 45.5% in 2024, supported by robust rare disease research infrastructure, favorable reimbursement frameworks, and orphan drug support policies, with the U.S. witnessing notable adoption driven by early diagnosis and regulatory incentives
- Asia-Pacific is expected to be the fastest growing region in the lysosomal acid lipase deficiency treatment market during the forecast period due to growing healthcare access, improved metabolic disorder awareness, and expanding newborn screening programs
- Enzyme replacement therapy (ERT) segment dominated the lysosomal acid lipase deficiency treatment market with a market share of 67.8% in 2024, owing to its proven ability to address the root cause of the disease and improve clinical outcomes across both pediatric and adult patient populations
Report Scope and Lysosomal Acid Lipase Deficiency Treatment Market Segmentation
|
Attributes |
Lysosomal Acid Lipase Deficiency Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lysosomal Acid Lipase Deficiency Treatment Market Trends
Advancements in Enzyme Replacement and Precision Therapies
- A prominent trend in the global LAL deficiency treatment market is the continuous advancement in enzyme replacement therapies (ERT) and the emergence of precision treatment strategies aimed at improving clinical outcomes and patient adherence in both pediatric and adult populations
- For instance, sebelipase alfa (Kanuma), the first and only FDA-approved ERT for LAL deficiency, has set a benchmark in treatment, offering improved liver function, reduced lipid accumulation, and increased survival, especially in infants with Wolman disease
- Current developments are focused on optimizing dosing schedules, enhancing bioavailability, and reducing immunogenic reactions, which remain a concern in long-term ERT. Researchers are also exploring next-generation formulations with extended half-life and reduced infusion burden
- The trend is further supported by growing interest in precision medicine approaches such as genetic profiling and biomarker-guided treatment to better identify candidates for therapy, monitor disease progression, and tailor interventions to individual patient needs
- Moreover, several biopharmaceutical firms are investing in novel therapeutic approaches, including gene therapy and mRNA platforms, with the potential to offer long-lasting or curative solutions by addressing the root genetic defect of the disorder
- This shift towards innovative, patient-centered therapeutic options is transforming expectations in the LAL deficiency space and paving the way for a more sustainable and effective long-term disease management landscape
Lysosomal Acid Lipase Deficiency Treatment Market Dynamics
Driver
Increased Diagnosis Rates and Supportive Regulatory Landscape
- The rising global awareness of rare genetic and metabolic diseases, along with improvements in diagnostic technologies such as next-generation sequencing (NGS), is significantly contributing to earlier and more accurate diagnosis of LAL deficiency
- For instance, expanded newborn screening programs in the U.S. and Europe are leading to earlier detection of LAL deficiency, allowing for timely intervention with enzyme replacement therapy and improved clinical outcomes
- Regulatory bodies such as the FDA and EMA have granted orphan drug status and priority review to LAL deficiency treatments, which has incentivized pharmaceutical companies to invest in R&D. These regulatory incentives, along with favorable reimbursement policies in key markets, are driving faster approval timelines and broader treatment access
- The increasing advocacy from patient organizations and rare disease networks is also playing a pivotal role in raising awareness, supporting research funding, and improving patient access to approved therapies particularly in high-income countries with structured rare disease frameworks
Restraint/Challenge
High Treatment Costs and Limited Access in Developing Region
- Despite therapeutic advancements, the high cost of enzyme replacement therapies such as sebelipase alfa remains a significant barrier to market growth, particularly in developing economies where healthcare systems face funding constraints
- For instance, the annual treatment cost for LAL deficiency can exceed USD 300,000 per patient, making it financially challenging for healthcare providers and payers in low- and middle-income countries to offer widespread access
- In addition, limited disease awareness among healthcare professionals and lack of diagnostic infrastructure in many regions contribute to underdiagnosis or misdiagnosis, thereby restricting treatment uptake
- Reimbursement delays, absence of dedicated rare disease policies, and a lack of local manufacturing capacity for biopharmaceuticals further constrain access to treatment
- Overcoming these hurdles will require collaborative efforts, including price negotiations, government subsidies, and partnerships between global health organizations and pharma companies to improve access and affordability in underserved regions
Lysosomal Acid Lipase Deficiency Treatment Market Scope
The market is segmented on the basis of treatment, therapy type, indication, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the lysosomal acid lipase deficiency treatment market is segmented into enzyme replacement therapy (ERT), lipid modifying agents (statins), surgery, and supportive care. The enzyme replacement therapy segment dominated the market with the largest revenue share of 67.8% in 2024, owing to the proven efficacy of sebelipase alfa (Kanuma) in improving survival rates and reducing liver and cardiovascular complications. ERT is currently the only disease-specific treatment available, making it the cornerstone of therapy for both Wolman disease and cholesteryl ester storage disease (CESD).
The lipid modifying agents segment, particularly statins, is expected to witness modest growth during forecast period as an adjunct therapy, helping to manage lipid profiles in milder cases or in regions with limited access to ERT. However, their limited disease-modifying capability keeps them in a supportive rather than primary role.
- By Therapy Type
On the basis of therapy type, the lysosomal acid lipase deficiency treatment market is segmented into liver transplant and hematopoietic stem cell transplant (HSCT). Liver transplant holds a notable market share, primarily used in severe cases where liver damage is irreversible and ERT is either unavailable or insufficient. It remains a last-resort treatment due to associated surgical risks and limited organ availability.
Hematopoietic stem cell transplant is expected to witness fastest growth during forecast period, as it has been explored in experimental or severe pediatric cases. Its role remains investigational and limited by high risks and low success rates, though it may see potential in future with emerging gene-editing advancements.
- By Indication
On the basis of indication, the lysosomal acid lipase deficiency treatment market is segmented into Wolman disease and cholesteryl ester storage disease (CESD). The Wolman disease segment accounted for a dominant share in 2024 due to its more severe and life-threatening nature, which demands immediate and aggressive treatment such as ERT shortly after birth.
The CESD segment is anticipated to grow steadily through 2032, as increasing awareness and improved diagnostic access help identify this more chronic, adult-onset form of LAL deficiency, leading to broader treatment uptake.
- By End-Users
On the basis of end-users, the lysosomal acid lipase deficiency treatment market is segmented into hospitals, specialty clinics, and others. Hospitals dominated the market in 2024, holding the highest revenue share due to their role in diagnosis, initiation of ERT, and management of complications. These facilities are best equipped with multidisciplinary teams essential for treating rare metabolic diseases.
Specialty clinics are expected to witness the fastest growth during the forecast period, driven by the emergence of rare disease centers offering specialized care, genetic counseling, and personalized treatment pathways for LAL deficiency patients.
- By Distribution Channel
On the basis of distribution channel, the lysosomal acid lipase deficiency treatment market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, and others. Hospital pharmacies held the largest market share in 2024, driven by the fact that most ERT treatments are administered intravenously under medical supervision in clinical settings.
Online pharmacies are expected to grow at a rapid pace during forecast period, due to increasing digital health adoption, rising patient convenience, and expanding cold-chain logistics capabilities for biologic drug delivery in select regions.
Lysosomal Acid Lipase Deficiency Treatment Market Regional Analysis
- North America dominated the lysosomal acid lipase deficiency treatment market with the largest revenue share of 45.5% in 2024, supported by robust rare disease research infrastructure, favorable reimbursement frameworks, and orphan drug support policies, with the U.S. witnessing notable adoption driven by early diagnosis and regulatory incentives
- The region benefits from favorable reimbursement frameworks, proactive newborn screening programs, and significant investment in orphan drug development by leading biopharmaceutical companies
- The U.S., in particular, accounts for the majority of regional demand due to its robust regulatory incentives, early access to innovative treatments, and the presence of specialized care centers, positioning North America as the primary hub for LAL deficiency management across both pediatric and adult populations
U.S. Lysosomal Acid Lipase Deficiency Treatment Market Insight
The U.S. lysosomal acid lipase deficiency treatment market captured the largest revenue share of 83% in North America in 2024, driven by a well-established rare disease framework, robust reimbursement systems, and early access to enzyme replacement therapies such as sebelipase alfa. High levels of disease awareness among healthcare providers, strong genetic screening infrastructure, and the presence of leading pharmaceutical innovators contribute to the country’s leadership. In addition, the U.S. benefits from proactive patient advocacy networks and clinical research initiatives supporting earlier diagnosis and personalized treatment approaches.
Europe Lysosomal Acid Lipase Deficiency Treatment Market Insight
The Europe lysosomal acid lipase deficiency treatment market is projected to grow at a steady CAGR throughout the forecast period, driven by national rare disease plans, supportive regulatory incentives, and expansion of newborn screening programs. Countries such as Germany, France, and the U.K. are investing in improved genetic testing and diagnostic protocols, enabling early intervention. Increased government funding and pan-European collaborations in rare disease research also support therapy accessibility. The presence of major biopharma companies and clinical research organizations further contributes to market growth across both Western and Central Europe.
U.K. Lysosomal Acid Lipase Deficiency Treatment Market Insight
The U.K. lysosomal acid lipase deficiency treatment market is expected to grow at a notable CAGR during the forecast period, driven by the country’s centralized National Health Service (NHS), which facilitates early diagnosis, rare disease coordination, and subsidized treatment. The integration of LAL deficiency into newborn screening pilots and genetic testing panels supports earlier detection. In addition, orphan drug approvals by the MHRA and the growing number of metabolic disease specialists are promoting increased access to advanced therapies, further strengthening the market outlook.
Germany Lysosomal Acid Lipase Deficiency Treatment Market Insight
The Germany lysosomal acid lipase deficiency treatment market is poised to expand steadily, backed by strong healthcare infrastructure, high spending on rare disease management, and the availability of innovative therapies. Germany’s emphasis on precision medicine and its proactive approach to genetic counseling have improved LAL deficiency diagnosis and care delivery. National reimbursement pathways and patient registries aid in streamlining treatment access. As clinical trial activity in rare metabolic disorders increases, Germany is expected to emerge as a key research and treatment hub in the region.
Asia-Pacific Lysosomal Acid Lipase Deficiency Treatment Market Insight
The Asia-Pacific lysosomal acid lipase deficiency treatment market is projected to grow at the fastest CAGR of 21.8% from 2025 to 2032, driven by improving healthcare access, increasing rare disease awareness, and rapid diagnostic advancements in countries such as China, Japan, and India. Growing government investment in genetic testing and newborn screening initiatives is facilitating earlier detection. Moreover, rising interest from global biopharma firms in tapping into the region’s large patient pool, along with expanding orphan drug policies, is opening up new growth opportunities.
Japan Lysosomal Acid Lipase Deficiency Treatment Market Insight
The Japan lysosomal acid lipase deficiency treatment market is gaining traction due to its advanced healthcare system, strong governmental focus on rare disease management, and increasing availability of genetic testing. The country’s aging population and emphasis on early diagnosis are contributing to increased identification and treatment of LAL deficiency cases. Regulatory support from the PMDA and the inclusion of sebelipase alfa under national insurance coverage are aiding in therapy uptake. Japan is also emerging as a leader in regional clinical trials and precision treatment development.
India Lysosomal Acid Lipase Deficiency Treatment Market Insight
The India lysosomal acid lipase deficiency treatment market accounted for the largest revenue share in Asia Pacific in 2024 due to its expanding healthcare infrastructure, growing middle class, and rising awareness of genetic disorders. While access to enzyme replacement therapies remains limited in certain areas, the increasing rollout of affordable diagnostic tools and government-backed rare disease policies are enhancing early identification. The rise of domestic pharmaceutical capabilities, collaborations with international biotech firms, and expansion of newborn screening programs position India as a key emerging market for LAL deficiency treatment.
Lysosomal Acid Lipase Deficiency Treatment Market Share
The lysosomal acid lipase deficiency treatment industry is primarily led by well-established companies, including:
- Alexion Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Lysosomal Acid Lipase Deficiency Treatment Market?
- In March 2025, the PEARL trial at UCSF received FDA clearance to administer prenatal enzyme replacement therapy (ERT) using sebelipase alfa for fetuses diagnosed with Wolman disease. This pioneering strategy aims to initiate treatment in utero, potentially reducing immune response-related complications and improving survival outcomes compared to postnatal therapy
- In January 2025, the FAHA’s PAN Foundation launched a new LAL‑D Premium Fund, offering up to USD 4,700 per year to eligible patients to help cover their health insurance premiums. This financial assistance program aims to reduce the economic burden for those undergoing lifelong treatments such as sebelipase alfa
- In December 2024, researchers published practical guidelines in Nutrients for the diagnosis and management of LAL-D, with a particular focus on Wolman disease. These consensus recommendations, developed by clinical experts, provide standardized protocols to facilitate earlier diagnosis and optimize patient care across various healthcare settings
- In June 2024, a pivotal preclinical study published in Nature Communications demonstrated the potential of adeno-associated virus (AAV8)-mediated gene therapy to cure lysosomal acid lipase deficiency (LAL-D) in mouse models. The gene therapy corrected liver function and normalized lipid metabolism, indicating a promising alternative to chronic enzyme replacement therapy (ERT). This development highlights the growing momentum toward gene-based, long-term curative treatments for rare metabolic diseases
- In April 2024, the U.K.'s National Institute for Health and Care Excellence (NICE) issued final guidance recommending sebelipase alfa (Kanuma) for treating infants with rapidly progressive LAL-D (Wolman disease), provided treatment begins before the age of two. The recommendation was based on strong clinical evidence demonstrating improved survival and liver function in early-treated patients. This marks a critical step in improving access to life-saving therapy for pediatric patients with rare lipid storage disorders
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.