Global Pseudohypoaldosteronism Type 1 Market
Market Size in USD Million
CAGR :
% 
 USD
30.50 Million
USD
44.38 Million
2024
2032
USD
30.50 Million
USD
44.38 Million
2024
2032
| 2025 –2032 | |
| USD 30.50 Million | |
| USD 44.38 Million | |
|
|
|
|
Pseudohypoaldosteronism Type 1 Market Size
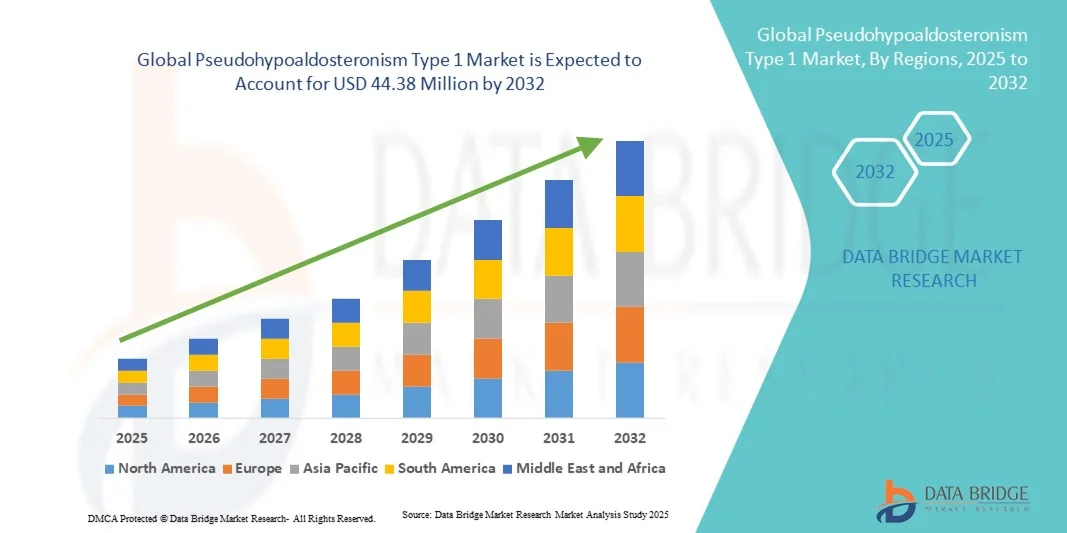
- The global Pseudohypoaldosteronism Type 1 market size was valued at USD 30.50 million in 2024 and is expected to reach USD 44.38 million by 2032, at a CAGR of 4.80% during the forecast period
- The market growth is largely fueled by the rising prevalence of rare genetic disorders, advancements in genetic testing, and development of targeted therapies, which are enhancing early diagnosis and personalized treatment approaches
- Furthermore, increasing awareness among healthcare professionals and patients, coupled with technological advancements in diagnostics and treatment options, is driving the adoption of PHA1 therapies, thereby significantly boosting the industry's growth
Pseudohypoaldosteronism Type 1 Market Analysis
- Pseudohypoaldosteronism Type 1 (PHA1), a rare genetic disorder characterized by salt wasting and resistance to mineralocorticoids, is increasingly recognized in pediatric and adult healthcare due to its impact on growth, electrolyte balance, and overall health, making early diagnosis and effective management critical for patient outcomes
- The escalating demand for PHA1 treatments is primarily fueled by advancements in genetic testing and diagnostic technologies, increased awareness among healthcare professionals, and growing emphasis on rare disease management, which together facilitate timely intervention and personalized therapy approaches
- North America dominated the PHA1 market with the largest revenue share of 41.7% in 2024, attributed to high healthcare expenditure, strong research and development capabilities, and the presence of key pharmaceutical players actively focusing on rare disease therapies, with the U.S. witnessing significant advancements in treatment protocols and genetic testing initiatives
- Asia-Pacific is expected to be the fastest-growing region in the PHA1 market during the forecast period, with a projected CAGR of 6.2%, driven by increasing healthcare infrastructure, rising awareness programs, and government support for rare disease diagnosis and treatment initiatives
- The sodium supplement segment dominated the PHA1 treatment market in 2024 with a market share of 45%, driven by its established efficacy in managing electrolyte imbalances and maintaining normal growth and development in affected patients
Report Scope and Pseudohypoaldosteronism Type 1 Market Segmentation
|
Attributes |
Pseudohypoaldosteronism Type 1 Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Pseudohypoaldosteronism Type 1 Market Trends
Advancements in Genetic Testing and Early Diagnosis
- A significant and accelerating trend in the PHA1 market is the increasing adoption of advanced genetic testing technologies that allow for early and accurate diagnosis, improving patient outcomes and enabling personalized treatment approaches
- For instance, next-generation sequencing (NGS) panels can detect mutations in the NR3C2 and SCNN1 genes associated with PHA1, facilitating timely intervention and better management of electrolyte imbalances
- Genetic testing integration in clinical practice enables earlier identification of at-risk neonates and children, reducing complications from delayed diagnosis and improving long-term growth and development
- The seamless integration of advanced diagnostics with pediatric and rare disease care programs allows clinicians to manage treatment plans more efficiently and monitor patient response over time
- This trend towards more precise, technology-driven diagnosis is fundamentally reshaping treatment strategies, with companies such as Blueprint Genetics developing comprehensive PHA1 testing solutions for clinical use
- The demand for rapid and reliable genetic diagnostics is growing across both developed and emerging markets, as healthcare providers increasingly prioritize proactive rare disease management
Pseudohypoaldosteronism Type 1 Market Dynamics
Driver
Rising Need for Effective Treatment and Rare Disease Management
- The increasing prevalence of PHA1 cases, coupled with growing awareness of rare genetic disorders, is a significant driver for the heightened demand for effective therapies and clinical management solutions
- For instance, pediatric hospitals and specialty clinics are expanding screening and treatment programs to manage electrolyte imbalances and prevent severe complications in PHA1 patients
- As clinicians become more aware of the importance of early intervention, therapies such as sodium supplementation and hormone replacement are being increasingly adopted to improve patient outcomes
- Furthermore, rising government initiatives and funding for rare disease management are making diagnosis and treatment more accessible in developed and emerging regions
- The availability of comprehensive treatment protocols, patient support programs, and improved access to specialized care are key factors propelling the adoption of PHA1 management solutions in clinical practice
- Growing patient advocacy and support groups are raising awareness, facilitating earlier diagnosis, and influencing policy decisions to improve treatment availability
- Increasing investment in R&D for novel therapies and drug formulations is expected to expand treatment options and improve clinical outcomes for PHA1 patients
Restraint/Challenge
Limited Awareness and High Cost of Genetic Testing
- Limited awareness among general practitioners and caregivers about PHA1 and its clinical management poses a significant challenge to broader market penetration and timely treatment initiation
- For instance, delayed recognition of symptoms such as dehydration and growth retardation often results in late diagnosis and increased risk of complications in affected children
- Addressing this challenge requires extensive education programs, clinical training, and awareness campaigns to improve understanding of PHA1 among healthcare providers and caregivers
- In addition, the relatively high cost of advanced genetic testing and specialized therapies can be a barrier for patients in developing regions or underinsured populations, limiting access to early diagnosis and treatment
- Overcoming these challenges through increased awareness, reimbursement support, and cost-effective diagnostic solutions will be vital for sustained growth of the PHA1 market
- Regulatory hurdles and lack of standardized treatment guidelines in some regions can delay adoption of novel therapies and diagnostic tools
- Limited availability of specialized healthcare centers in rural or underdeveloped regions further restricts patient access to timely PHA1 management and follow-up care
Pseudohypoaldosteronism Type 1 Market Scope
The market is segmented on the basis of type, diagnosis, treatment, end-users, and distribution channel.
- By Type
On the basis of type, the PHA1 market is segmented into recessive and dominant forms. The recessive segment dominated the market with the largest revenue share in 2024, driven by its higher prevalence in certain populations and the severity of clinical manifestations that necessitate early diagnosis and long-term management. Patients with recessive PHA1 often require continuous monitoring of electrolyte levels and intensive therapy to prevent complications such as dehydration, failure to thrive, and recurrent hospitalizations. The segment benefits from established treatment protocols and growing awareness among pediatricians and endocrinologists. In addition, diagnostic advancements such as genetic testing panels are widely applied in recessive cases, enhancing early intervention. Hospitals and specialty clinics frequently prioritize recessive PHA1 management due to the complexity of care required, which also increases the adoption of sodium supplementation and hormone therapy. The segment also attracts research initiatives focusing on gene-targeted therapies due to the severe clinical needs.
The dominant segment is anticipated to witness the fastest growth during the forecast period, attributed to improved detection methods and rising awareness of milder, late-onset cases that were historically underdiagnosed. Dominant PHA1 cases often present less severe symptoms, encouraging earlier outpatient management rather than intensive hospital care. The segment’s growth is further supported by increasing research in gene therapy and personalized medicine approaches. Growing screening initiatives and educational programs for healthcare providers are expected to drive adoption, while diagnostic improvements allow more families to identify and manage dominant PHA1 effectively. Expanded awareness campaigns and patient registries also contribute to the faster adoption of this type.
- By Diagnosis
On the basis of diagnosis, the PHA1 market is segmented into blood test, plasma test, and others. The blood test segment dominated the market in 2024, accounting for the largest revenue share, due to its established accuracy in detecting electrolyte imbalances and hormone levels critical for confirming PHA1. Blood tests are often the first-line diagnostic tool in pediatric and neonatal care, enabling prompt clinical intervention. Their widespread availability in hospitals and specialty clinics ensures accessibility for most patients. The segment also benefits from integration with genetic testing workflows to confirm NR3C2 or SCNN1 mutations. Regular blood testing is essential for ongoing management, making it a key revenue contributor in the PHA1 treatment pathway. Hospitals rely on blood tests for monitoring treatment efficacy and adjusting therapy.
The plasma test segment is expected to witness the fastest growth from 2025 to 2032, driven by advancements in sensitive plasma assays that allow for more precise monitoring of aldosterone and renin activity. Plasma tests provide real-time insights into disease progression and therapy efficacy, facilitating individualized treatment adjustments. They are increasingly preferred in research-oriented hospitals and specialty centers for long-term monitoring. The segment growth is further fueled by rising adoption in regions expanding rare disease diagnostic infrastructure and the increasing demand for minimally invasive testing. Integration with telehealth services allows remote monitoring, which is boosting growth in emerging regions.
- By Treatment
On the basis of treatment, the PHA1 market is segmented into sodium supplements and others. The sodium supplements segment dominated the market in 2024 with the largest revenue share of 45%, due to its established efficacy in correcting electrolyte imbalances and preventing severe dehydration episodes in patients. Sodium supplementation remains the cornerstone of therapy in both recessive and dominant PHA1 cases, often combined with careful dietary management and regular monitoring. Hospitals and specialty clinics routinely prescribe tailored sodium regimens to maintain normal growth and development. The segment also benefits from established supply chains and inclusion in rare disease treatment guidelines, ensuring consistent demand. Its long-standing clinical acceptance contributes to continued dominance in the market.
The “others” treatment segment, including hormone therapy and emerging targeted interventions, is expected to witness the fastest growth during the forecast period, driven by ongoing R&D in precision therapies and biologics. This segment offers potential improvements in patient quality of life and reduced hospitalization rates. Adoption is increasing in specialized clinics offering personalized medicine approaches. Technological advancements in drug delivery and monitoring are further boosting growth, especially in markets with expanding rare disease healthcare infrastructure. Increasing awareness of novel therapies and clinical trial participation also supports rapid adoption.
- By End-Users
On the basis of end-users, the PHA1 market is segmented into hospitals, specialty clinics, and others. The hospitals segment dominated the market in 2024, accounting for the largest revenue share, due to the high requirement for comprehensive care and advanced monitoring facilities that hospitals provide for PHA1 patients. Hospitals are central to acute management, long-term monitoring, and integration of diagnostic and treatment services. They also have greater access to specialized therapies and laboratory testing, supporting continuous patient care. The segment benefits from government funding and insurance coverage policies that favor institutional care for rare diseases. Hospitals frequently collaborate with research organizations to improve treatment outcomes. The high patient volume and complex case management contribute to the dominance of this segment.
The specialty clinics segment is expected to witness the fastest growth from 2025 to 2032, attributed to the rising number of centers focused on rare disease management, genetic counseling, and outpatient care. Clinics provide personalized monitoring, patient education, and access to emerging therapies, which is especially important for dominant PHA1 patients with milder symptoms. The expansion of specialized pediatric and endocrine clinics globally is driving growth. Integration with telemedicine and digital health platforms allows remote patient management, enhancing clinic adoption. Increased awareness campaigns by rare disease organizations also support segment growth.
- By Distribution Channel
On the basis of distribution channel, the PHA1 market is segmented into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment dominated the market in 2024, due to its ability to provide continuous access to sodium supplements and hormone therapies directly to patients under supervised care. Hospital pharmacies facilitate proper dosing, monitoring, and counseling, ensuring adherence and reducing complications. They also act as a central hub for rare disease management, integrating diagnostics and treatment supply. The segment benefits from institutional purchasing policies and government support for rare disease care. Hospitals can efficiently manage inventory for chronic therapy requirements. Strong collaboration with pharmaceutical manufacturers reinforces the segment’s dominance.
The online pharmacy segment is expected to witness the fastest growth from 2025 to 2032, driven by increasing digital adoption, convenience for patients in remote regions, and subscription-based medicine delivery models. Online platforms enhance access to specialized supplements and medications, particularly for patients requiring long-term therapy. Growth is also supported by telehealth services, increasing patient engagement, and the expanding e-commerce infrastructure in emerging markets. The availability of doorstep delivery and digital prescription services further accelerates adoption. Patient preference for convenience and recurring supply subscriptions boosts segment expansion.
Pseudohypoaldosteronism Type 1 Market Regional Analysis
- North America dominated the PHA1 market with the largest revenue share of 41.7% in 2024, attributed to high healthcare expenditure, strong research and development capabilities, and the presence of key pharmaceutical players actively focusing on rare disease therapies, with the U.S. witnessing significant advancements in treatment protocols and genetic testing initiatives
- Healthcare providers and hospitals in the region prioritize early diagnosis and treatment of PHA1, supported by widespread availability of genetic testing, specialized pediatric care, and advanced monitoring facilities
- This strong adoption is further supported by government initiatives, insurance coverage for rare diseases, and the presence of leading pharmaceutical and diagnostic companies, establishing North America as a key market hub for PHA1 management
U.S. Pseudohypoaldosteronism Type 1 Market Insight
The U.S. PHA1 market captured the largest revenue share of 45% in 2024 within North America, driven by advanced healthcare infrastructure and high awareness of rare genetic disorders. Hospitals and specialty clinics are prioritizing early diagnosis and management through genetic testing and regular monitoring of electrolytes. The growing emphasis on rare disease treatment programs, coupled with insurance coverage and government support, is fueling adoption. Moreover, the increasing integration of genetic diagnostics, personalized treatment plans, and patient support programs is significantly contributing to market expansion. The availability of clinical trials and research collaborations also accelerates the uptake of novel therapies.
Europe Pseudohypoaldosteronism Type 1 Market Insight
The Europe PHA1 market is projected to expand at a substantial CAGR during the forecast period, primarily driven by comprehensive healthcare systems and increasing awareness of rare disease management. Countries such as Germany, France, and Italy are witnessing growth in pediatric and specialty clinics equipped with advanced diagnostic tools. The rise in urban healthcare facilities and government initiatives promoting rare disease treatment programs is encouraging adoption. European healthcare providers are increasingly integrating genetic testing and hormone therapy into routine patient care. The region also sees significant expansion across hospital and specialty clinic segments.
U.K. Pseudohypoaldosteronism Type 1 Market Insight
The U.K. PHA1 market is anticipated to grow at a noteworthy CAGR, driven by rising awareness of early diagnosis and proactive rare disease management. Increasing support from patient advocacy groups and government funding is encouraging both hospitals and specialty clinics to adopt genetic testing and personalized treatment solutions. Concerns regarding electrolyte imbalance complications in children are prompting proactive intervention strategies. The integration of genetic diagnostics with electronic health records enhances monitoring and follow-up care. The availability of clinical trial programs and advanced treatment protocols further stimulates market growth.
Germany Pseudohypoaldosteronism Type 1 Market Insight
The Germany PHA1 market is expected to expand at a considerable CAGR during the forecast period, fueled by rising awareness of rare genetic disorders and well-developed healthcare infrastructure. Hospitals and specialty clinics are increasingly adopting genetic testing and hormone therapy for early diagnosis and management. Germany’s focus on research and innovation promotes development and uptake of advanced treatment protocols. The integration of pediatric care programs with genetic counseling is increasing patient accessibility. Local government initiatives supporting rare disease management further drive adoption. High standards of care and specialized clinical expertise encourage faster market penetration.
Asia-Pacific Pseudohypoaldosteronism Type 1 Market Insight
The Asia-Pacific PHA1 market is poised to grow at the fastest CAGR of 6.2% during the forecast period of 2025 to 2032, driven by expanding healthcare infrastructure, rising awareness programs, and government support for rare disease diagnosis and treatment. Countries such as India, China, and Japan are witnessing increasing adoption of genetic testing and specialized pediatric care for early diagnosis. The growing number of specialty clinics and telemedicine integration improves patient accessibility. Awareness campaigns and collaboration with global pharmaceutical companies are promoting adoption. Rising disposable incomes and healthcare investments further propel market growth.
Japan Pseudohypoaldosteronism Type 1 Market Insight
The Japan PHA1 market is gaining momentum due to advanced healthcare systems, high awareness of rare diseases, and government-supported genetic screening programs. Hospitals and specialty clinics are increasingly integrating hormone therapy and sodium supplementation for patient management. The country’s emphasis on pediatric care and preventive healthcare promotes early diagnosis. Adoption of digital health platforms and telemedicine improves patient monitoring and follow-up care. Collaborative research and clinical trial activities enhance availability of advanced therapies. Japan’s aging population and focus on personalized medicine are such asly to drive continued market growth.
India Pseudohypoaldosteronism Type 1 Market Insight
The India PHA1 market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to expanding healthcare infrastructure, growing awareness of rare diseases, and increasing adoption of genetic testing. Hospitals and specialty clinics are actively providing diagnostic and treatment services, particularly in urban centers. Government initiatives supporting rare disease management, along with rising disposable incomes, are driving market expansion. The push towards digital health and telemedicine services improves access in semi-urban and rural areas. Availability of affordable treatment options and collaboration with domestic and global pharmaceutical companies further fuels adoption. Increasing patient education and awareness programs also contribute to market growth.
Pseudohypoaldosteronism Type 1 Market Share
The Pseudohypoaldosteronism Type 1 industry is primarily led by well-established companies, including:
- Sandoz AG (Switzerland)
- Pfizer Inc. (U.S.)
- Hikma Pharmaceuticals PLC (U.K.)
- Merck & Co., Inc. (U.S.)
- Blueprint Genetics Oy (Finland)
- PreventionGenetics (U.S.)
- Breda Genetics (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- GeneDx ,LLC (U.S.)
- Invitae Corporation (U.S.)
- Eurofins Scientific (Luxembourg)
- Ambry Genetics (U.S.)
- Fulgent Genetics (U.S.)
- Quest Diagnostics (U.S.)
- Thermo Fisher Scientific, Inc (U.S.)
- Illumina, Inc. (U.S.)
- PerkinElmer (U.S.)
- Labcorp (U.S.)
What are the Recent Developments in Global Pseudohypoaldosteronism Type 1 Market?
- In September 2025, A study investigated the functional implications of the γENaC G532S mutation in PHA1, contributing to the understanding of genetic variations in the disease. This research enhances the knowledge of genetic factors influencing PHA1 and may inform future diagnostic and therapeutic strategies
- In August 2025, A case report detailed the early molecular confirmation of systemic PHA1 and the management of hyperkalemia using sodium polystyrene sulfonate (SPS). This approach underscores the importance of early genetic diagnosis and tailored treatments in managing systemic PHA1
- In August 2025, A study reported a case where a child had both Graves' disease and PHA1D, highlighting the complexity of diagnosing and managing multiple endocrine disorders simultaneously. This underscores the importance of comprehensive clinical evaluation in patients with overlapping symptoms
- In July 2025, A case report highlighted the challenges in diagnosing PHA1, as a 3-month-old infant was initially misdiagnosed with congenital adrenal hyperplasia. This emphasizes the need for accurate differential diagnosis and awareness among healthcare providers to ensure appropriate treatment
- In June 2025, Research identified reduced sodium-dependent liquid absorption in the lungs as a pulmonary manifestation of PHA1B, leading to various respiratory symptoms. This finding broadens the clinical understanding of PHA1B and may influence future management approaches
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.