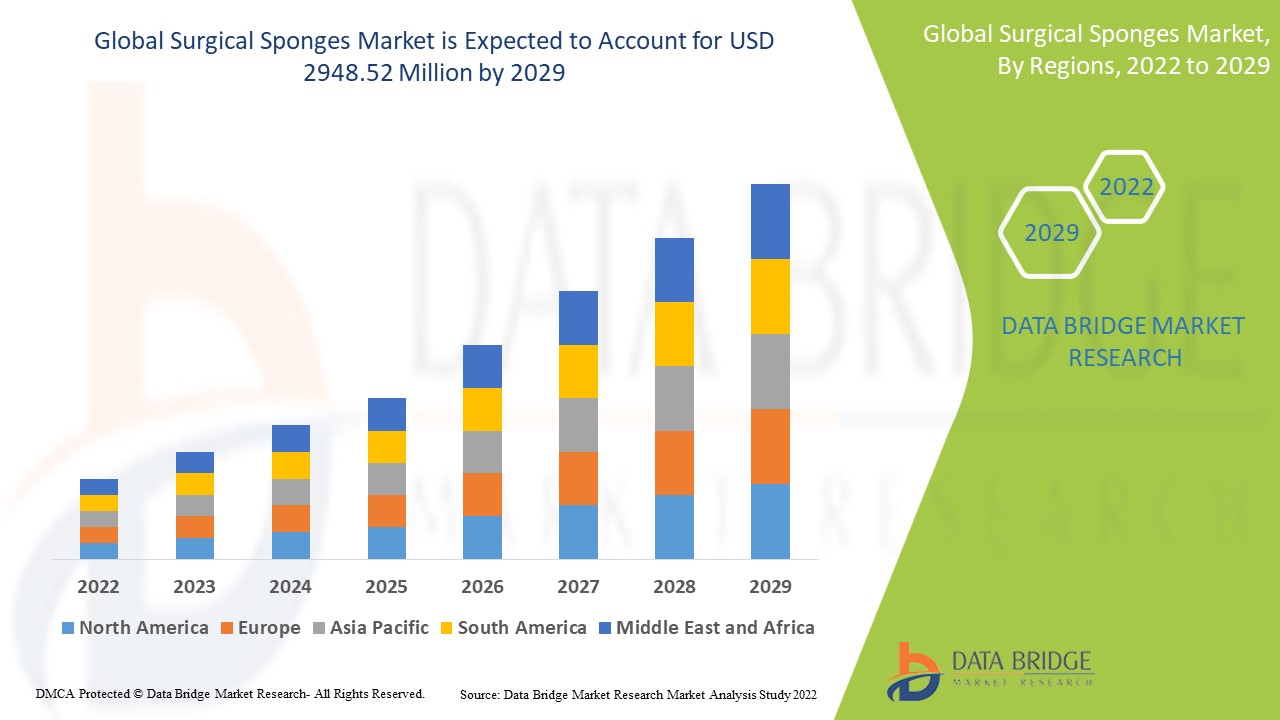
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SURGICAL SPONGES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 ARRIVING AT THE GLOBAL SURGICAL SPONGES MARKET
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMPANY POSITIONING GRID
2.2.5 COMAPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 TOP TO BOTTOM ANALYSIS
2.2.8 STANDARDS OF MEASUREMENT
2.2.9 VENDOR SHARE ANALYSIS
2.2.10 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.11 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL SURGICAL SPONGES MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.2 PATIENT FLOW DIAGRAM
5.3 KEY PRICING STRATEGIES
5.4 KEY PATIENT ENROLLMENT STRATEGIES
5.5 INTERVIEWS WITH LAB TECHNICIAN
5.6 INTERVIEWS WITH BLOOD BANK SPECALIST
5.7 INTERVIEWS WITH GENERAL PHYSICIAN
5.8 INTERVIEWS WITH GENERAL SURGEON
5.9 OTHER KOL SNAPSHOTS
6 GLOBAL SURGICAL SPONGES MARKET, BY PRODUCT
7 (*NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS )
7.1 OVERVIEW
7.2 X-RAY DETECTABLE SPONGES
7.2.1 8 IN. X 4 IN.
7.2.1.1. STERILE
7.2.1.1.1. BANDED
7.2.1.1.1.1 12 PACK SIZE
7.2.1.1.1.1.1. MARKET VALUE (USD MILLION)
7.2.1.1.1.1.2. MARKET VOLUME (UNITS)
7.2.1.1.1.1.3. AVERAGE SELLING PRICE (USD)
7.2.1.1.1.2 24 PACK SIZE
7.2.1.1.1.2.1. MARKET VALUE (USD MILLION)
7.2.1.1.1.2.2. MARKET VOLUME (UNITS)
7.2.1.1.1.2.3. AVERAGE SELLING PRICE (USD)
7.2.1.1.1.3 0THERS
7.2.1.1.2. UNBANDED
7.2.1.1.2.1 12 PACK SIZE
7.2.1.1.2.1.1. MARKET VALUE (USD MILLION)
7.2.1.1.2.1.2. MARKET VOLUME (UNITS)
7.2.1.1.2.1.3. AVERAGE SELLING PRICE (USD)
7.2.1.1.2.2 24 PACK SIZE
7.2.1.1.2.2.1. MARKET VALUE (USD MILLION)
7.2.1.1.2.2.2. MARKET VOLUME (UNITS)
7.2.1.1.2.2.3. AVERAGE SELLING PRICE (USD)
7.2.1.1.2.3 0THERS
7.2.1.2. NON-STERILE
7.2.2 4 IN. X 4 IN.
7.2.2.1. STERILE
7.2.2.1.1. BANDED
7.2.2.1.1.1 12 PACK SIZE
7.2.2.1.1.2 24 PACK SIZE
7.2.2.1.1.3 0THERS
7.2.2.1.2. UNBANDED
7.2.2.1.2.1 12 PACK SIZE
7.2.2.1.2.2 24 PACK SIZE
7.2.2.1.2.3 0THERS
7.2.3 4 IN. X 3 ½ IN
7.2.3.1. STERILE
7.2.3.1.1. BANDED
7.2.3.1.1.1 12 PACK SIZE
7.2.3.1.1.2 24 PACK SIZE
7.2.3.1.1.3 0THERS
7.2.3.1.2. UNBANDED
7.2.3.1.2.1 12 PACK SIZE
7.2.3.1.2.2 24 PACK SIZE
7.2.3.1.2.3 0THERS
7.2.4 OTHEES
7.3 COTTON GAUZE SPONGES
7.3.1 8-PLY
7.3.1.1. NON-STERILE
7.3.1.1.1. 2 IN. X 2 IN
7.3.1.1.1.1 12 PACK SIZE
7.3.1.1.1.2 24 PACK SIZE
7.3.1.1.1.3 0THERS
7.3.1.1.2. 4 IN. X 4 IN
7.3.1.1.2.1 12 PACK SIZE
7.3.1.1.2.2 24 PACK SIZE
7.3.1.1.2.3 0THERS
7.3.1.1.3. OTHERS
7.3.1.2. STERILE
7.3.1.2.1. 2 IN. X 2 IN
7.3.1.2.1.1 12 PACK SIZE
7.3.1.2.1.2 24 PACK SIZE
7.3.1.2.1.3 0THERS
7.3.1.2.2. 4 IN. X 4 IN
7.3.1.2.2.1 12 PACK SIZE
7.3.1.2.2.2 24 PACK SIZE
7.3.1.2.2.3 0THERS
7.3.1.2.3. OTHERS
7.3.2 12-PLY
7.3.2.1. NON-STERILE
7.3.2.1.1. 2 IN. X 2 IN
7.3.2.1.1.1 12 PACK SIZE
7.3.2.1.1.2 24 PACK SIZE
7.3.2.1.1.3 0THERS
7.3.2.1.2. 4 IN. X 4 IN
7.3.2.1.2.1 12 PACK SIZE
7.3.2.1.2.2 24 PACK SIZE
7.3.2.1.2.3 0THERS
7.3.2.1.3. OTHERS
7.3.2.2. STERILE
7.3.2.2.1. 2 IN. X 2 IN
7.3.2.2.1.1 12 PACK SIZE
7.3.2.2.1.2 24 PACK SIZE
7.3.2.2.1.3 0THERS
7.3.2.2.2. 4 IN. X 4 IN
7.3.2.2.2.1 12 PACK SIZE
7.3.2.2.2.2 24 PACK SIZE
7.3.2.2.2.3 0THERS
7.3.2.2.3. OTHERS
7.3.3 16-PLY
7.3.3.1. NON-STERILE
7.3.3.1.1. 2 IN. X 2 IN
7.3.3.1.1.1 12 PACK SIZE
7.3.3.1.1.2 24 PACK SIZE
7.3.3.1.1.3 0THERS
7.3.3.1.2. 4 IN. X 4 IN
7.3.3.1.2.1 12 PACK SIZE
7.3.3.1.2.2 24 PACK SIZE
7.3.3.1.2.3 0THERS
7.3.3.1.3. OTHERS
7.3.3.2. STERILE
7.3.3.2.1. 2 IN. X 2 IN
7.3.3.2.1.1 12 PACK SIZE
7.3.3.2.1.2 24 PACK SIZE
7.3.3.2.1.3 0THERS
7.3.3.2.2. 4 IN. X 4 IN
7.3.3.2.2.1 12 PACK SIZE
7.3.3.2.2.2 24 PACK SIZE
7.3.3.2.2.3 0THERS
7.3.3.2.3. OTHERS
7.3.4 OTHERS
7.4 LAPAROTOMY SPONGES
7.4.1 BY SIZE
7.4.1.1. 10 CM X 40 CM
7.4.1.2. 10 CM X 45 CM
7.4.1.3. 20 CM X 20 CM
7.4.1.4. OTHER
7.4.2 BY MESH
7.4.2.1. 12 X 8
7.4.2.2. 15X 11
7.4.2.3. OTHERS
7.4.3 BY STERILITY
7.4.3.1. STERILIE
7.4.3.2. NON STERILIE
7.4.4 OTHERS
7.5 NONWOVEN SPONGES
7.5.1 8-PLY
7.5.1.1. NON-STERILE
7.5.1.1.1. 2 IN. X 2 IN
7.5.1.1.1.1 12 PACK SIZE
7.5.1.1.1.2 24 PACK SIZE
7.5.1.1.1.3 0THERS
7.5.1.1.2. 4 IN. X 4 IN
7.5.1.1.2.1 12 PACK SIZE
7.5.1.1.2.2 24 PACK SIZE
7.5.1.1.2.3 0THERS
7.5.1.1.3. OTHERS
7.5.1.2. STERILE
7.5.1.2.1. 2 IN. X 2 IN
7.5.1.2.1.1 12 PACK SIZE
7.5.1.2.1.2 24 PACK SIZE
7.5.1.2.1.3 0THERS
7.5.1.2.2. 4 IN. X 4 IN
7.5.1.2.2.1 12 PACK SIZE
7.5.1.2.2.2 24 PACK SIZE
7.5.1.2.2.3 0THERS
7.5.1.2.3. OTHERS
7.5.2 12-PLY
7.5.2.1. NON-STERILE
7.5.2.1.1. 2 IN. X 2 IN
7.5.2.1.1.1 12 PACK SIZE
7.5.2.1.1.2 24 PACK SIZE
7.5.2.1.1.3 0THERS
7.5.2.1.2. 4 IN. X 4 IN
7.5.2.1.2.1 12 PACK SIZE
7.5.2.1.2.2 24 PACK SIZE
7.5.2.1.2.3 0THERS
7.5.2.1.3. OTHERS
7.5.2.2. STERILE
7.5.2.2.1. 2 IN. X 2 IN
7.5.2.2.1.1 12 PACK SIZE
7.5.2.2.1.2 24 PACK SIZE
7.5.2.2.1.3 0THERS
7.5.2.2.2. 4 IN. X 4 IN
7.5.2.2.2.1 12 PACK SIZE
7.5.2.2.2.2 24 PACK SIZE
7.5.2.2.2.3 0THERS
7.5.2.2.3. OTHERS
7.5.3 16-PLY
7.5.3.1. NON-STERILE
7.5.3.1.1. 2 IN. X 2 IN
7.5.3.1.1.1 12 PACK SIZE
7.5.3.1.1.2 24 PACK SIZE
7.5.3.1.1.3 0THERS
7.5.3.1.2. 4 IN. X 4 IN
7.5.3.1.2.1 12 PACK SIZE
7.5.3.1.2.2 24 PACK SIZE
7.5.3.1.2.3 0THERS
7.5.3.1.3. OTHERS
7.5.3.2. STERILE
7.5.3.2.1. 2 IN. X 2 IN
7.5.3.2.1.1 12 PACK SIZE
7.5.3.2.1.2 24 PACK SIZE
7.5.3.2.1.3 0THERS
7.5.3.2.2. 4 IN. X 4 IN
7.5.3.2.2.1 12 PACK SIZE
7.5.3.2.2.2 24 PACK SIZE
7.5.3.2.2.3 0THERS
7.5.3.2.3. OTHERS
7.5.4 OTHERS
7.6 OTHERS
8 GLOBAL SURGICAL SPONGES MARKET, BY MATERIAL
8.1 OVERVIEW
8.2 COTTON
8.2.1 ROUND
8.2.2 SPHERICAL
8.2.3 CYLINDRICAL
8.2.4 OTHERS
8.3 CELLULOSE
8.3.1 ROUND
8.3.2 SPHERICAL
8.3.3 CYLINDRICAL
8.3.4 OTHERS
8.4 PVA
8.4.1 ROUND
8.4.2 SPHERICAL
8.4.3 CYLINDRICAL
8.4.4 OTHERS
8.5 POLYURETHANE
8.5.1 ROUND
8.5.2 SPHERICAL
8.5.3 CYLINDRICAL
8.5.4 OTHERS
8.6 RAYON
8.6.1 ROUND
8.6.2 SPHERICAL
8.6.3 CYLINDRICAL
8.6.4 OTHERS
8.7 OTHERS
9 GLOBAL SURGICAL SPONGES MARKET, BY STERILITY TYPE
9.1 OVERVIEW
9.2 STERILE SURGICAL SPONGE
9.3 NON-STERILE SURGICAL SPONGE
10 GLOBAL SURGICAL SPONGES MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 GENERAL SURGERY
10.3 NEUROSURGERY
10.4 LAPAROTOMY
10.5 DENTAL SURGERY
10.6 ENT SURGERY
10.7 OTHERS
11 GLOBAL SURGICAL SPONGES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICS
11.4 PATHOLOGY LABORATORIES
11.5 BLOOD BANKS
11.6 RESEARCH & ACADEMIC LABORATORIES
11.7 HOME DIAGNOSTICS
11.8 OTHERS
12 GLOBAL SURGICAL SPONGES MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 REATAIL SALE
12.4 OTHERS
13 GLOBAL SURGICAL SPONGES MARKET, BY GEOGRAPHY
13.1 GLOBAL SURGICAL SPONGES MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
13.2 NORTH AMERICA
13.2.1 U.S.
13.2.1.1. U.S. SURGICAL SPONGES MARKET, BY PRODUCT
13.2.1.2. U.S. SURGICAL SPONGES MARKET, BY METHOD
13.2.1.3. U.S. SURGICAL SPONGES MARKET, BY TYPE OF SURGICAL SPONGES METHOD
13.2.1.4. U.S. SURGICAL SPONGES MARKET, BY APPLICATION
13.2.1.5. U.S. SURGICAL SPONGES MARKET, BY END USERS
13.2.1.6. U.S. SURGICAL SPONGES MARKET, BY DISTRIBUTION CHANNEL
13.2.1.7. CANADA
13.2.1.8. MEXICO
13.3 EUROPE
13.3.1 GERMANY
13.3.2 U.K.
13.3.3 ITALY
13.3.4 FRANCE
13.3.5 SPAIN
13.3.6 SWITZERLAND
13.3.7 NETHERLANDS
13.3.8 BELGIUM
13.3.9 RUSSIA
13.3.10 TURKEY
13.3.11 REST OF EUROPE
13.4 ASIA-PACIFIC
13.4.1 JAPAN
13.4.2 CHINA
13.4.3 SOUTH KOREA
13.4.4 INDIA
13.4.5 AUSTRALIA
13.4.6 SINGAPORE
13.4.7 THAILAND
13.4.8 INDONESIA
13.4.9 MALAYSIA
13.4.10 PHILIPPINES
13.4.11 REST OF ASIA-PACIFIC
13.5 SOUTH AMERICA
13.5.1 BRAZIL
13.5.2 REST OF SOUTH AMERICA
13.6 MIDDLE EAST AND AFRICA
13.6.1 SOUTH AFRICA
13.6.2 REST OF MIDDLE EAST AND AMERICA
14 GLOBAL SURGICAL SPONGES MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14.5 MERGERS & ACQUISITIONS
14.6 NEW PRODUCT DEVELOPMENT & APPROVALS
14.7 EXPANSIONS & PARTNERSHIP
14.8 REGULATORY CHANGES
15 COMPANY PROFILE
15.1 BD
15.1.1 COMPANY OVERVIEW
15.1.2 GEOGRAPHIC PRESENCE
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENTS
15.2 MEDTRONIC
15.2.1 COMPANY OVERVIEW
15.2.2 GEOGRAPHIC PRESENCE
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENTS
15.3 MOLNLYCKE
15.3.1 COMPANY OVERVIEW
15.3.2 GEOGRAPHIC PRESENCE
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENTS
15.4 BSN MEDICAL
15.4.1 COMPANY OVERVIEW
15.4.2 GEOGRAPHIC PRESENCE
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENTS
15.5 MEDLINE
15.5.1 COMPANY OVERVIEW
15.5.2 GEOGRAPHIC PRESENCE
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENTS
15.6 CARDINAL HEALTH
15.6.1 COMPANY OVERVIEW
15.6.2 GEOGRAPHIC PRESENCE
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENTS
15.7 HARTMANN
15.7.1 COMPANY OVERVIEW
15.7.2 GEOGRAPHIC PRESENCE
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENTS
15.8 LOHMANN & RAUSCHER
15.8.1 COMPANY OVERVIEW
15.8.2 GEOGRAPHIC PRESENCE
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENTS
15.9 AHLSTROM
15.9.1 COMPANY OVERVIEW
15.9.2 GEOGRAPHIC PRESENCE
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 OWENS & MINOR
15.10.1 COMPANY OVERVIEW
15.10.2 GEOGRAPHIC PRESENCE
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENTS
15.11 MCKESSON
15.11.1 COMPANY OVERVIEW
15.11.2 GEOGRAPHIC PRESENCE
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.12 ZHENDE MEDICAL
15.12.1 COMPANY OVERVIEW
15.12.2 GEOGRAPHIC PRESENCE
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 WINNER MEDICAL
15.13.1 COMPANY OVERVIEW
15.13.2 GEOGRAPHIC PRESENCE
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 JIANERKANG
15.14.1 COMPANY OVERVIEW
15.14.2 GEOGRAPHIC PRESENCE
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 HAKUZO
15.15.1 COMPANY OVERVIEW
15.15.2 GEOGRAPHIC PRESENCE
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENTS
15.16 DEROYAL
15.16.1 COMPANY OVERVIEW
15.16.2 GEOGRAPHIC PRESENCE
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENTS
15.17 ALLMED MEDICAL
15.17.1 COMPANY OVERVIEW
15.17.2 GEOGRAPHIC PRESENCE
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENTS
15.18 ASC
15.18.1 COMPANY OVERVIEW
15.18.2 GEOGRAPHIC PRESENCE
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENTS
15.19 CROSSTEX
15.19.1 COMPANY OVERVIEW
15.19.2 GEOGRAPHIC PRESENCE
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 KETTENBACH
15.20.1 COMPANY OVERVIEW
15.20.2 GEOGRAPHIC PRESENCE
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENTS
15.21 DUKAL
15.21.1 COMPANY OVERVIEW
15.21.2 GEOGRAPHIC PRESENCE
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENTS
15.22 TEXPOL
15.22.1 COMPANY OVERVIEW
15.22.2 GEOGRAPHIC PRESENCE
15.22.3 PRODUCT PORTFOLIO
15.22.4 RECENT DEVELOPMENTS
15.23 MEDICOM
15.23.1 COMPANY OVERVIEW
15.23.2 REVENUE ANALYSIS
15.23.3 GEOGRAPHIC PRESENCE
15.23.4 PRODUCT PORTFOLIO
15.23.5 RECENT DEVELOPMENTS
15.24 GOLDWIN MEDICARE
15.24.1 COMPANY OVERVIEW
15.24.2 GEOGRAPHIC PRESENCE
15.24.3 PRODUCT PORTFOLIO
15.24.4 RECENT DEVELOPMENTS
15.25 CARDINAL HEALTH
15.25.1 COMPANY OVERVIEW
15.25.2 REVENUE ANALYSIS
15.25.3 GEOGRAPHIC PRESENCE
15.25.4 PRODUCT PORTFOLIO
15.25.5 RECENT DEVELOPMENTS
15.26 NIPRO MEDICAL CORPORATION
15.26.1 COMPANY OVERVIEW
15.26.2 GEOGRAPHIC PRESENCE
15.26.3 PRODUCT PORTFOLIO
15.26.4 RECENT DEVELOPMENTS
15.27 VOGT MEDICAL
15.27.1 COMPANY OVERVIEW
15.27.2 GEOGRAPHIC PRESENCE
15.27.3 PRODUCT PORTFOLIO
15.27.4 RECENT DEVELOPMENTS
15.28 INNVOL
15.28.1 COMPANY OVERVIEW
15.28.2 REVENUE ANALYSIS
15.28.3 GEOGRAPHIC PRESENCE
15.28.4 PRODUCT PORTFOLIO
15.28.5 RECENT DEVELOPMENTS
15.29 TERUMO MEDICAL CORPORATION
15.29.1 COMPANY OVERVIEW
15.29.2 REVENUE ANALYSIS
15.29.3 GEOGRAPHIC PRESENCE
15.29.4 PRODUCT PORTFOLIO
15.29.5 RECENT DEVELOPMENTS
15.3 MCKESSON MEDICAL-SURGICAL INC.
15.30.1 COMPANY OVERVIEW
15.30.2 REVENUE ANALYSIS
15.30.3 GEOGRAPHIC PRESENCE
15.30.4 PRODUCT PORTFOLIO
15.30.5 RECENT DEVELOPMENTS
16 RELATED REPORTS
17 CONCLUSION
18 QUESTIONNAIRE
19 ABOUT DATA BRIDGE MARKET RESEARCH