COVID-19 Impact on Biosimilar in Healthcare Industry
The outbreak of Coronavirus highlights the need of effective treatment option in order to get rid of such pandemic. Various types of drugs have been validated for COVID-19 treatment among which biosimilar drugs are getting more attention.
The biosimilar drug market is growing even during the COVID-19 pandemic and this is due to the increasing demand of emergency used drugs worldwide to treat COVID-19 patients. The sales of biosimilar drugs are increasing due to increasing demand of biosimilar drugs for treatment of rheumatoid arthritis, Crohn’s disease, among others.
TABLE 1. SALES DATA OF BIOSIMILAR DRUG PROJECTED FOR 2019/2024
|
COMPANY NAME
|
BIOSIMILAR
|
U.S. SALES IN 2019/24
|
|
Celltrion/Pfizer
|
Inflectra
|
300/430
|
|
Mylan/Biocon
|
Fulphila
|
224/255
|
|
Cohreus Biosciences
|
Udencya
|
356/498
|
|
Pfizer
|
Retacrit
|
141/101
|
|
Amgen/Allergan
|
Mvasi
|
121/305
|
Biosimilar drug market is also growing due to increasing number of clinical trials in order to demonstrate the safety and efficacy of these drugs.
PRICE IMPACT
The demand of effective treatment for various kinds of diseases such as rheumatoid arthritis, diabetes among others is increasing during COVID-19 pandemic due to fear among people regarding COVID-19 infection.
The price of biosimilar drugs has increased during the COVID-19 pandemic as no any generic competitive drug is still available, along with increasing demand of safe and effective drug.
For instance,
- The price of adalimumab, manufactured by the AbbVie has increased to 7.4% in January, 2020. Moreover, the price of etanercept has also increased to 16.5% during the same year as no competitive drug is available in the market.
- The price of tocilizumab is increasing in India during the COVID-19 pandemic as the demand of effective biosimilar is on great surge. The another factor for increasing demand is presence of few tocilizumab manufacturer, this is due to the fact that only Roche and its subsidiary Genentech makes the tocilizumab and has not licensed it out to any other company. In India, this drug is accessible to pharmacies among other distribution channel via only Indian generic drug company that is Cipla which led to the high price of tocilizumab in India.
This thus signifies that due to COVID-19 pandemic the price of biosimilar used for treatment of inflammatory symptoms among others caused by Coronavirus increased.
IMPACT ON DEMAND
The Coronavirus led to the widespread closure of local manufacturing plants and companies and has badly affected the various region of world. Due to extensive lockdown and isolation the economic activity has affected adversely which has impacted the global economic activity.
COVID-19 is expected to be an opportunity for biosimilar market as biosimilar drugs have been proved to effective against various kinds of diseases such as autoimmune disorders, blood disorders, and infectious diseases, among others.
For instance,
- In December 2019, WHO prequalified the trastuzumab, the first biosimilar drug for breast cancer. This is a monoclonal antibody and has shown high efficacy in curing early stage breast cancer, moreover has also proved effective against the more advanced form of diseases. As the cancer patients get more concerned during the COVID-19 pandemic the demand of effective biosimilar is also increasing along with other treatment options.
FIGURE 1. APPROXIMATE NUMBER OF BREAST CANCER CASES IN 2018, INDIA, FEMALES, ALL AGES
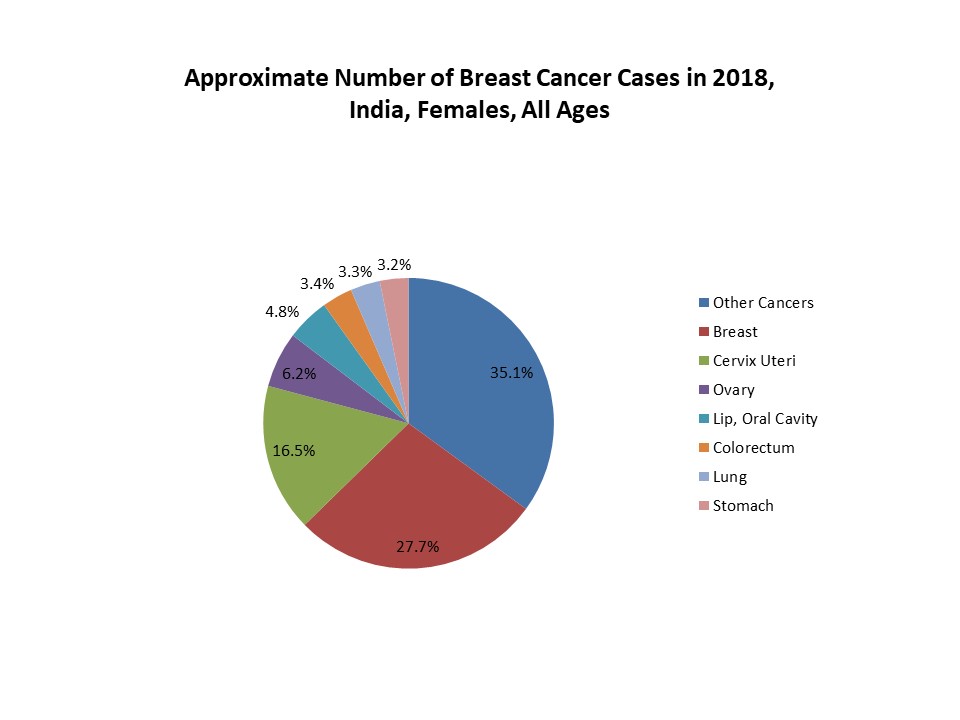 FIGURE 2. PERCENTAGE OF NEW CANCER CASES AMONG WOMEN IN 2020, CANADA
FIGURE 2. PERCENTAGE OF NEW CANCER CASES AMONG WOMEN IN 2020, CANADA
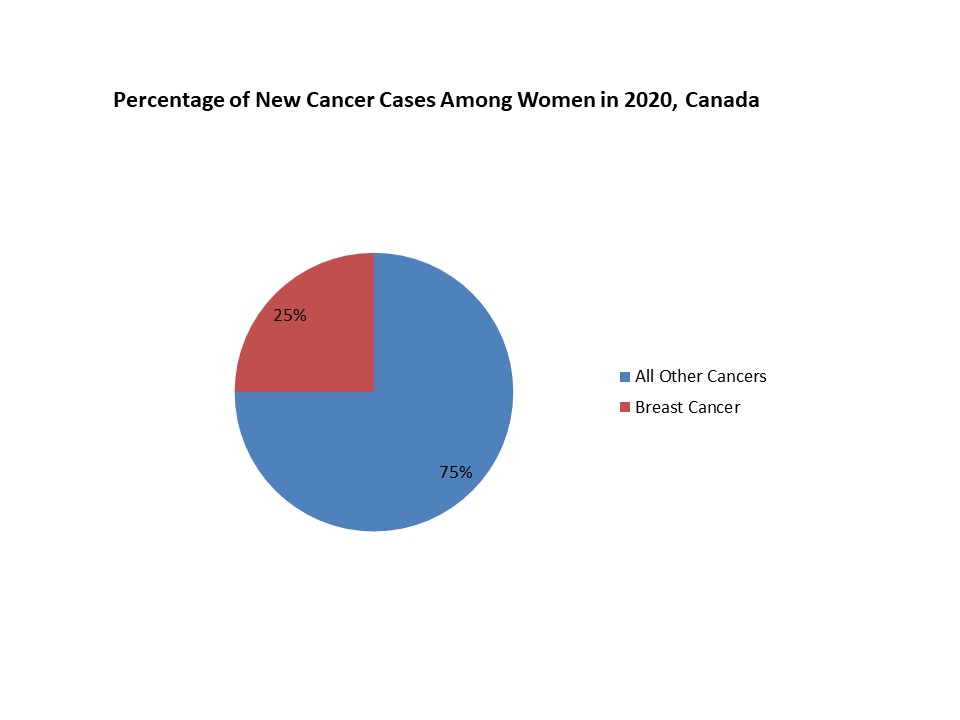 Above figure suggests that the number of breast cancer cases is rising among women all over the world which enhanced the demand of effective biosimilar treatment option, thus, increasing number of breast cancer cases led to increased demand of biosimilar drugs.
Above figure suggests that the number of breast cancer cases is rising among women all over the world which enhanced the demand of effective biosimilar treatment option, thus, increasing number of breast cancer cases led to increased demand of biosimilar drugs.
Moreover the increasing cases of COVID-19 have also paved the way for enhanced demand of biosimilars.
For instance,
- In September 2020, Ministry of Health of the Russian Federation recommended the usage of Ilsira, a monoclonal antibody which works by blocking the IL-6 receptor for treatment of severe complications in COVID-19 patients. This drug has been proved effective in preventing the cytokine storm during Coronavirus infections.
- In July 2020, Biocon received approval for its biosimilar Itolizumab for treatment of COVID-19 patients. This is the first anti CD6 biologic therapy which has been approved for treatment of moderate to severe complications of COVID-19. This thus, suggested that increasing cases of COVID-19 led to the development of effective biosimilar drug in order to achieve safe treatment.
- As per the news of April 2020, this has been depicted that Kevzara (Sarilumab), a monoclonal antibody developed by Regeneron and Sanofi has the potential to inhibit the IL-6 pathway and hence clinical trials have been initiated in order to prove its efficacy for COVID-19 treatment.
- In India the demand for tocilizumab enhanced as the demand of effective COVID-19 drug increased. Tocilizumab, if used correctly can prevent the serious damage to their lungs.
- In March 2020, China approved the Roche arthritis drug Tocilizumab for some COVID-19 patients. As per the report this drug is sold under the name Actemra by Roche and can be prescribed to Coronavirus patients who have reported to have serious lung damage and inflammatory condition due enhanced level of IL-6 protein.
This thus signifies that increasing prevalence of COVID-19 poses a life threatening effect to various types of patients already suffering from several kinds of disease among the most recent known COVID-19 pandemic.
Thus the demand of effective treatment option that is biosimilar drug has enhanced as have been proved to block IL-6 receptor which in turn reduces inflammatory affects. This thus demonstrates that COVID-19 is accelerating the demand of biosimilar market.
IMPACT ON SUPPLY
As COVID-19 spreading began in China the surge of medicines has been enhanced due to increasing patient’s volume which put a pressure over the supply of medicaments. Moreover decreased capacity of air freight carriers due to continuous travel ban across the globe has also led to shortage of drugs supply in the U.S., among other countries across the world.
However the market players are adopting several initiatives in order to maintain a continuous supply of biosimilar drugs.
For instance,
- As per the news of September 2020, it has been suggested that Sandoz is working closely with the Association for Accessible Medicines in order to enhance the global pharmaceutical supply chain.
- In September 2020, Eli Lilly and Company and Amgen collaborated in order for enhancing the global antibody manufacturing capacity that boosted the company’s supply capacity of COVID-19 therapies. As the company is involved in studying the potential of neutralizing antibodies for prevention and treatment of COVID-19, this initiative helped the company to enhance the supply capabilities of biosimilar drugs.
- As per the news of August 2020, mAbxience a company specialized in biosimilars stated that is continuously operating activities at the manufacturing plants of Munro in Argentina and León in Spain with all of its maximum capacity so as to guarantee the supply of biosimilars to consumers.
- According to the news of March 2020, Apotex stated that being the Canada’s largest pharmaceutical manufacturer the company is working diligently in order to minimize the COVID-19 impact on its supply chain of drugs involving biosimilar drugs, among others.
This suggests that increasing prevalence of COVID-19 poses threat for maintaining a continuous supply chain of biosimilar drug while initiative adopted by market players allows them to manage a continuous supply chain.
STRATEGIC DECISIONS OF MANUFACTURERS
Collaboration, agreements, strategic initiatives by market players such as Pfizer, Inc., Sandoz, Boehringer Ingelheim, Amega Biotech, mAbxience among others in the biosimilar market will help them to expand their product portfolio. This in turn will lead to increasing product sales and hence will enhance the overall company’s revenue.
Biosimilar manufacturing companies are taking so many strategic decisions in order to cope up with the current scenario of COVID-19 pandemic. The companies engaged in manufacturing of biosimilar are collaborating so as to accelerate the market growth.
Companies have already taken several kinds of strategic initiatives in order to cope up with the corona virus situation.
For instance,
- In June 2020, Pfizer Inc. received approval for NYVEPRIA, which is indicated to decrease the incidence of infection in patients suffering from non- myeloid malignancies. This approval helped the company to attain a lucrative growth even during the COVID-19 pandemic.
- In May 2020, Fresenius Kabi AG and medac signed a cooperative agreement in order to provide adalimumab biosimilar IDACIO for treatment of arthritis among other conditions. This partnership helped the companies to offer patients and doctors to access effective biosimilar therapies, thus this initiative enhanced the company’s revenue.
- In April 2020, mAbxience has inaugurated a new biosimilar monoclonal antibody manufacturing plant in Argentina. This is a large project and involved the investment of USD 40 million, this thus proved to be the significant milestone in the Latin America biopharma industry. These biosimilar drugs are used for treatment of autoimmune disease such as rheumatoid arthritis and oncological diseases. This initiative helped the company to enhance the manufacturing of biosimilar drugs which can be accessed by COVID-19 patients as well.
The increasing demand and increasing sales of biosimilar drugs are fueling the growth of biosimilar market. Thus, companies operating in the biosimilar market are adopting several strategies, including collaboration, agreements, market expansion to enhance their business. These strategic decisions by the market players helped them to attain a lucrative growth even during the COVID-19 pandemic.
CONCLUSION
As the pandemic of COVID-19 has resulted in several restrictions throughout the borders but still manufacturers of biosimilar are able to manage their stocks. Various manufacturers are continuously engage in conducting clinical trials for demonstrating the safety and efficacy of biosimilar along with this they are constantly monitoring the supply chain so as to attain a lucrative growth. Moreover, by raising price of biosimilar the companies are gaining extra profit which is helping them to combat the negative effect on overall revenue.