Endotoxintests sind für die Qualitätskontrolle von Arzneimitteln und Medizinprodukten unverzichtbar. Ihre Hauptaufgabe besteht in der Identifizierung und Quantifizierung von Endotoxinen – bakteriellen Nebenprodukten, die Medizinprodukte schädigen können. Fortschritte in diesem Bereich haben zu sensitiveren und schnelleren Testmethoden wie rekombinanten Faktor-C-Tests (RFC) geführt, die eine höhere Genauigkeit und kürzere Testzeiten bieten. Diese Innovationen gewährleisten die Sicherheit und Wirksamkeit von Gesundheitsprodukten, was für den Schutz der Patientengesundheit und die Einhaltung gesetzlicher Vorschriften von entscheidender Bedeutung ist.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/australia-endotoxin-testing-market
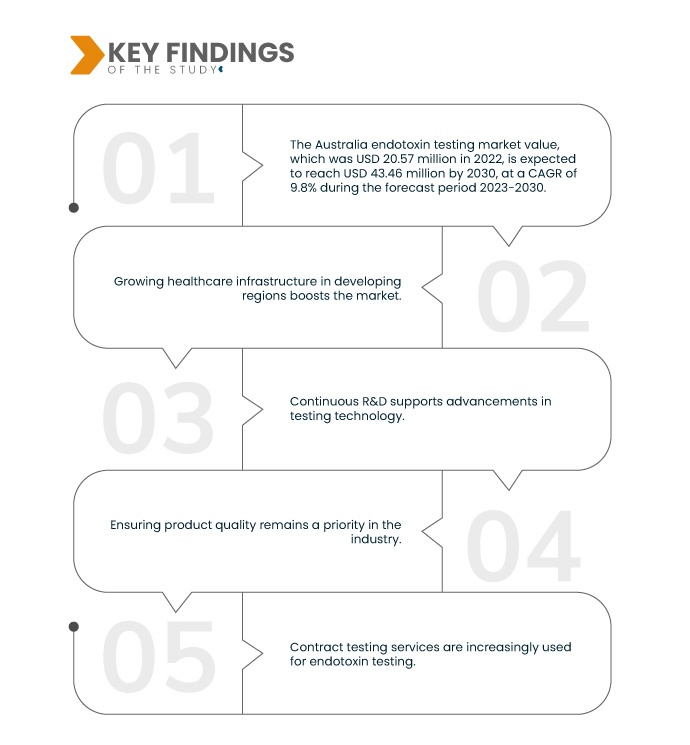
Data Bridge Market Research analysiert, dass der australische Markt für Endotoxintests , der im Jahr 2022 20,57 Millionen US-Dollar betrug, bis 2030 voraussichtlich 43,46 Millionen US-Dollar erreichen wird, was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 9,8 % im Prognosezeitraum 2023–2030 entspricht. Steigende Gesundheitsausgaben erfordern strenge Qualitätskontrollen bei der Herstellung medizinischer Produkte. Endotoxintests sind entscheidend, um die Sicherheit und Wirksamkeit von Arzneimitteln und Medizinprodukten zu gewährleisten und stehen im Einklang mit steigenden Gesundheitsausgaben und Patientensicherheitsbedenken.
Wichtigste Ergebnisse der Studie
Es wird erwartet, dass regulatorische Anforderungen das Marktwachstum vorantreiben
Strenge Vorschriften bilden einen Eckpfeiler der Arzneimittel- und Medizinprodukteproduktion. Sie schreiben Endotoxintests vor, um die Sicherheit dieser Produkte zu gewährleisten. Diese Vorschriften gewährleisten, dass die Endotoxinwerte innerhalb akzeptabler Grenzen liegen und Patienten nicht schädlichen Bakterien ausgesetzt werden. Die Einhaltung dieser Standards ist für die Produktzulassung und den Marktzugang unerlässlich, da sie die Qualität, Wirksamkeit und Sicherheit von Medizinprodukten gewährleistet und dem Engagement der Branche für höchste Gesundheitsstandards entspricht.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2023 bis 2030
|
Basisjahr
|
2022
|
Historische Jahre
|
2021 (Anpassbar auf 2015–2020)
|
Quantitative Einheiten
|
Umsatz in Millionen USD, Mengen in Einheiten, Preise in USD
|
Abgedeckte Segmente
|
Produkttyp (Endotoxin-Nachweiskits und -Reagenzien, Instrumente, Systeme und Software, Endotoxin-Testservices sowie Verbrauchsmaterialien und Zubehör), Testtyp (Limulus-Amöbozytenlysat-Test (LAL), Monozytenaktivierungstest (MAT), Kaninchen-Pyrogentest und rekombinanter C-Test (RFC), Anwendung (Pharmazeutische Herstellung, Herstellung medizinischer Geräte, Rohstoffproduktion und Verpackungsherstellung), Methode (Gel-Clot-Endotoxin-Test, chromogener Endotoxin-Test und turbidimetrischer Endotoxin-Test), Bezugsart (Große Gruppe, mittlere und kleine Gruppe und Einzelperson), Endbenutzer (Pharmazeutische Unternehmen, Biotechnologieunternehmen, Unternehmen für medizinische Geräte, Auftragsforschungsinstitute (CRO), Auftragshersteller (CMO), Akademische Forschungsinstitute und andere)
|
Abgedeckte Länder
|
China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Rest des asiatisch-pazifischen Raums (APAC).
|
Abgedeckte Marktteilnehmer
|
Eurofins Scientific (Frankreich), Thermo Fisher Scientific (USA), Pall Corporation. (USA), Lonza (Schweiz), Charles River Laboratories (USA), Merck KGaA (Deutschland), STERIS (Großbritannien), Société Générale de Surveillance SA (Frankreich), Sartorius AG (Deutschland) und GenScript (USA)
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse:
Der australische Markt für Endotoxintests ist nach Produkttyp, Testtyp, Anwendung, Methode, Kaufart und Endbenutzer segmentiert.
- Auf der Grundlage des Produkttyps ist der australische Markt für Endotoxintests in Endotoxin-Erkennungskits und -Reagenzien, Instrumente, Systeme und Software, Endotoxin-Testdienste sowie Verbrauchsmaterialien und Zubehör unterteilt.
- Auf der Grundlage des Testtyps ist der australische Markt für Endotoxintests in Limulus-Amöbozytenlysat-Test (LAL), Monozytenaktivierungstest (MAT), Kaninchen-Pyrogentest und rekombinanten C-Test (RFC) unterteilt.
- Auf der Grundlage der Anwendung ist der australische Markt für Endotoxintests in die Bereiche Arzneimittelherstellung, Herstellung medizinischer Geräte, Rohstoffproduktion und Verpackungsherstellung segmentiert.
- Auf der Grundlage der Methode ist der australische Markt für Endotoxintests in Gel-Clot-Endotoxin-Test, chromogenen Endotoxin-Test und turbidimetrischen Endotoxin-Test unterteilt.
- Auf der Grundlage der Kaufart ist der australische Markt für Endotoxintests in große Gruppen, mittlere und kleine Gruppen sowie Einzelpersonen segmentiert.
- Auf der Grundlage des Endverbrauchers ist der australische Markt für Endotoxintests in Pharmaunternehmen, Biotechnologieunternehmen, Medizingerätehersteller, Auftragsforschungsinstitute (CROs), Auftragshersteller (CMOs), akademische Forschungsinstitute und andere segmentiert.
Hauptakteure
Data Bridge Market Research erkennt die folgenden Unternehmen als Akteure auf dem australischen Markt für Endotoxintests an: Eurofins Scientific (Frankreich), Thermo Fisher Scientific (USA), Pall Corporation (USA), Lonza (Schweiz), Charles River Laboratories (USA), Merck KGaA (Deutschland),
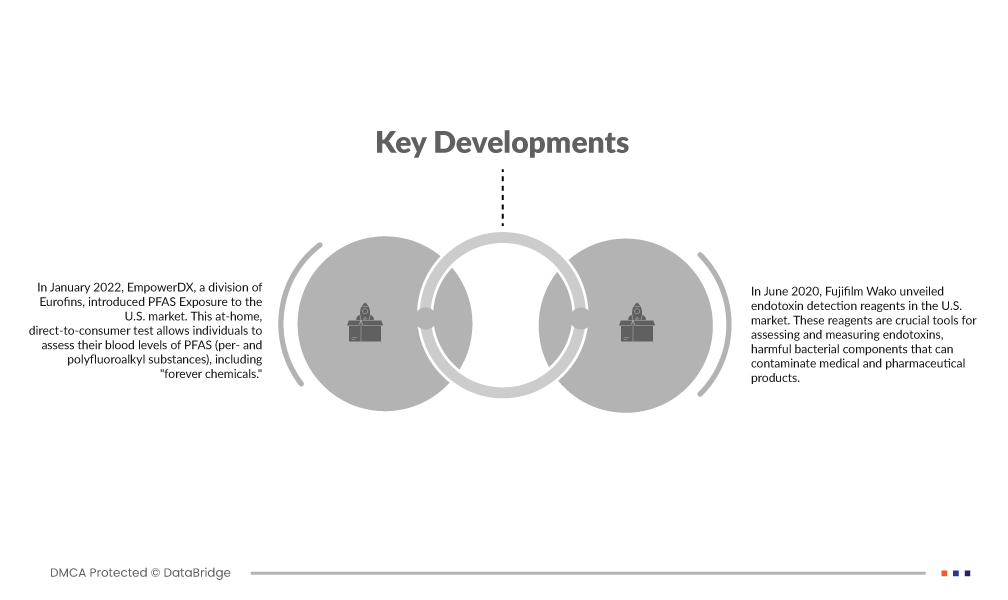
Marktentwicklungen
- Im Januar 2022 brachte EmpowerDX, ein Geschäftsbereich von Eurofins, den PFAS Exposure-Test auf den US-Markt. Dieser Test für zu Hause, direkt beim Verbraucher, ermöglicht es Einzelpersonen, ihren Blutspiegel von PFAS (per- und polyfluorierten Alkylsubstanzen), einschließlich sogenannter „ewiger Chemikalien“, zu bestimmen. PFAS Exposure bietet Verbrauchern eine bequeme Möglichkeit, ihre Belastung mit diesen potenziell schädlichen Verbindungen zu überwachen. Es gibt ihnen Einblick in ihre PFAS-Werte und fördert das Bewusstsein für Umwelt- und Gesundheitsprobleme.
- Im Juni 2020 brachte Fujifilm Wako Reagenzien zum Nachweis von Endotoxinen auf den US-Markt. Diese Reagenzien sind wichtige Instrumente zur Beurteilung und Messung von Endotoxinen – schädlichen Bakterienbestandteilen, die medizinische und pharmazeutische Produkte kontaminieren können. Der Markteintritt von Fujifilm Wako mit diesen Reagenzien trägt zur Sicherheit und Qualitätskontrolle von Gesundheits- und Pharmaprodukten bei, indem er präzise Endotoxintests ermöglicht und strengen Industriestandards und -vorschriften entspricht.
Für detailliertere Informationen zum australischen Marktbericht für Endotoxintests klicken Sie hier – https://www.databridgemarketresearch.com/reports/australia-endotoxin-testing-market