The high-cost medicines market in the Dominican Republic is experiencing a steady surge in demand driven by the increasing clinical burden of complex, chronic, and rare diseases, alongside the unmet treatment needs of underserved populations. As health infrastructure and diagnostic capabilities expand, more patients are being accurately diagnosed with conditions such as cancer, autoimmune disorders, hemophilia, multiple sclerosis, and other rare genetic diseases, many of which require targeted, high-cost therapies like biologics, monoclonal antibodies, or enzyme replacement drugs. This growing demand is further exacerbated by urban-rural disparities in access to specialty care, creating a dual challenge of increasing caseloads and uneven treatment coverage. As clinical practice evolves to address these complex conditions, the high-cost medicines market is becoming a critical component of the national response to non-communicable disease management, particularly in tertiary hospitals and specialized public programs.
The Dominican Republic is undergoing a pivotal transition in disease burden management—marked by higher detection rates of complex and rare conditions, growing clinical sophistication in tertiary care settings, and persistent healthcare access disparities in rural areas. These dynamics are converging to shape an urgent, sustained demand for high-cost medicines. Meeting this demand will require continued investment in diagnostics, equitable drug access, and training of specialist healthcare providers, alongside greater integration of underserved populations into public subsidy programs
Access Full Report @ https://www.databridgemarketresearch.com/reports/dominican-republic-high-cost-medicines-market
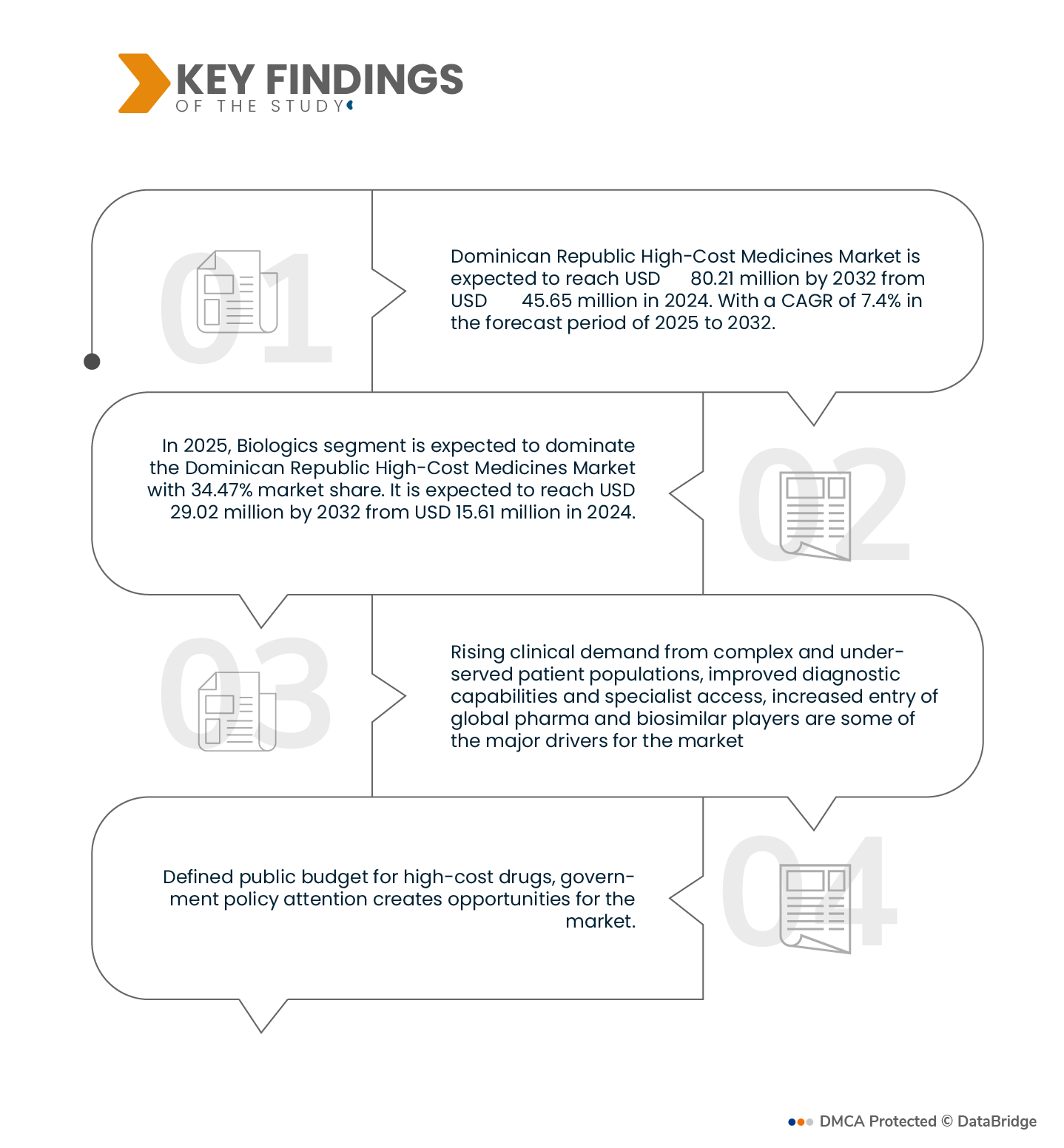
Data Bridge Market Research analyzes that the Dominican Republic High-Cost Medicines Market is expected to reach USD 80.21 Million by 2032 from USD 45.66 Million in 2024, growing at a substantial CAGR of 7.4% in the forecast period of 2025 to 2032.
Key Findings of the Study
Improved diagnostic capabilities and specialist access
The high‑cost medicines market in the Dominican Republic is being driven by steadily improving diagnostic infrastructure and greater access to specialized medical expertise. Enhanced cancer registries, expanded early detection programs, and the development of medical residency specialties are enabling earlier and more accurate diagnoses of conditions requiring expensive, targeted therapies. As hospitals and public health bodies invest in better laboratory, imaging, and pathology services, more patients are being identified earlier in their disease courses—making expensive high‑cost treatments clinically effective and financially justifiable. Meanwhile, specialist training programs in areas such as nephrology, dermatology, infectious diseases, and pediatric oncology are increasing the number of physicians capable of managing biologics, immunosuppressants, and other high‑cost interventions in both public and private sectors, acting as a key growth driver for the high‑cost medicines market.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2025 to 2032
|
|
Base Year
|
2024
|
|
Historic Year
|
2023 (Customizable 2018-2022)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
By Type (Biologics, Injectables & Infusions, Immunosuppressants, Specialty Pharmaceuticals, and Others), Therapy Type (Multi-Organ Support Systems, Respiratory Support, Cardio Vascular Support, Renal Support, and Hepatic (Liver) Support), End User (Hospitals, Specialized Treatment Centers, and Others), and Hospitals in High Cost Medicines Market By Type (Public and Private)
|
|
Countries Covered
|
Dominican Republic
|
|
Market Players Covered
|
Baxter (U.S.), Pfizer Inc. (U.S.), Takeda Pharmaceutical Company (Japan), Amgen Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), B. Braun SE (Germany), GSK plc. (U.K.), Alexion Pharmaceuticals, Inc (U.S.), AbbVie Inc. (U.S.), Astellas Pharma US, Inc. (Japan), Novartis AG (Switzerland), F. Hoffmann-La Roche Ltd (Switzerland), Bristol-Myers Squibb Company (U.S.), Sanofi S.A. (France), Grifols, S.A. (Spain) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
Dominican Republic High-Cost Medicines market is segmented into three notable segments which are based on Type, Therapy Type, and End user.
- On the basis of Type, Dominican Republic High-Cost Medicines market is segmented into Biologics, Injectables & Infusions, Immunosuppressants, Specialty Pharmaceuticals, and Others
In 2025, Biologics segment is expected to dominate the Dominican Republic High-Cost Medicines market
In 2025, Biologics segment is expected to dominate the market with a market share of 34.47% due to their increasing use in the treatment of complex and chronic conditions such as cancer, autoimmune disorders, and rare diseases, along with growing physician preference for targeted therapies that offer improved efficacy and patient outcomes. The dominance of biologics is further supported by government reimbursement programs like the Programa de Medicamentos de Alto Costo, which prioritize funding for high-impact therapies, as well as the rising availability of biosimilars that improve access and affordability within both public and private healthcare sectors.
- On the basis of Therapy Type, the Dominican Republic High-Cost Medicines market is segmented into Multi-Organ Support Systems, Respiratory Support, Cardio Vascular Support, Renal Support, and Hepatic (Liver) Support
In 2025, Multi-Organ Support Systems segment is expected to dominate the Dominican Republic High-Cost Medicines market
In 2025, Multi-Organ Support Systems segment is expected to dominate the market with a market share of 30.52% due to the increasing incidence of complex critical care cases involving sepsis, trauma, and multi-organ dysfunction syndrome (MODS), which require integrated and simultaneous support across multiple organ systems. This dominance is further driven by the growing adoption of advanced technologies such as Extracorporeal Membrane Oxygenation (ECMO) and Continuous Renal Replacement Therapy (CRRT), particularly in tertiary care and intensive care units. Government and private sector investments in upgrading critical care infrastructure, along with rising clinical expertise in managing multi-organ failure, have also contributed to this segment’s projected growth. Additionally, the centralized procurement of high-cost multi-organ support therapies under national health programs ensures broader accessibility and consistent demand across the healthcare system.
- On the basis of End User, the Dominican Republic High-Cost Medicines market is segmented into Hospitals, Specialized Treatment Centers, and Others. In 2025, the Hospitals segment is expected to dominate the market with a market share of 79.85%
Major Players
Data Bridge Market Research analyzes Baxter (U.S.), Pfizer Inc. (U.S.), Takeda Pharmaceutical Company (Japan), Amgen Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), B. Braun SE (Germany), GSK plc. (U.K.), Alexion Pharmaceuticals, Inc (U.S.), AbbVie Inc. (U.S.), Astellas Pharma US, Inc. (Japan), Novartis AG (Switzerland), F. Hoffmann-La Roche Ltd (Switzerland), Bristol-Myers Squibb Company (U.S.), Sanofi S.A. (France), Grifols, S.A. (Spain) among others as the major market players of the Dominican Republic High-Cost Medicines market.
Market Developments
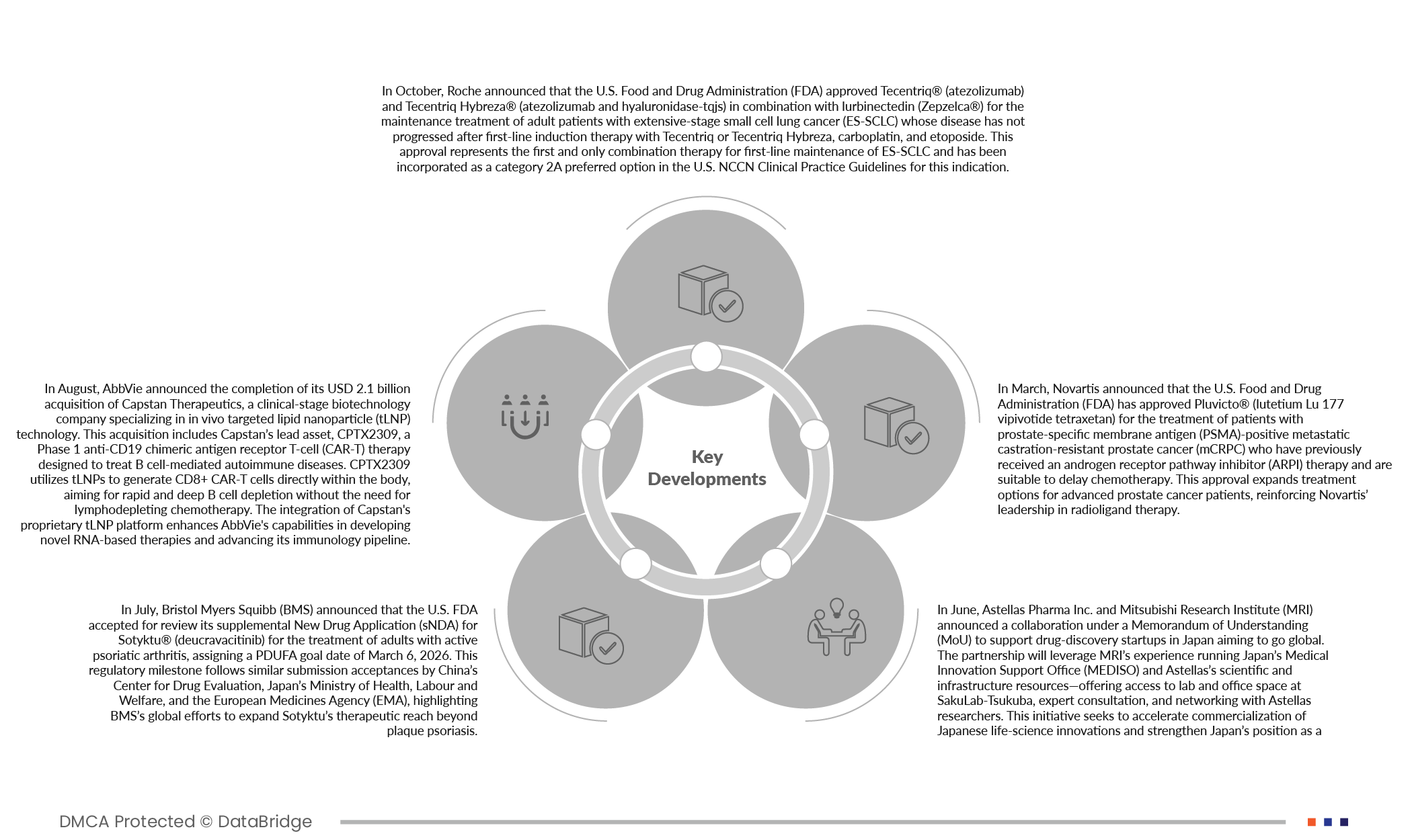
- In March 2025, Novartis announced that the U.S. Food and Drug Administration (FDA) has approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have previously received an androgen receptor pathway inhibitor (ARPI) therapy and are suitable to delay chemotherapy. This approval expands treatment options for advanced prostate cancer patients, reinforcing Novartis’ leadership in radioligand therapy.
- In October 2025, Roche announced that the U.S. Food and Drug Administration (FDA) approved Tecentriq (atezolizumab) and Tecentriq Hybreza (atezolizumab and hyaluronidase-tqjs) in combination with lurbinectedin (Zepzelca) for the maintenance treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) whose disease has not progressed after first-line induction therapy with Tecentriq or Tecentriq Hybreza, carboplatin, and etoposide. This approval represents the first and only combination therapy for first-line maintenance of ES-SCLC and has been incorporated as a category 2A preferred option in the U.S. NCCN Clinical Practice Guidelines for this indication.
- In June 2025, Roche announced that the European Commission (EC) approved a label extension for Evrysdi (risdiplam) to include a new, room-temperature stable 5mg tablet for people living with spinal muscular atrophy (SMA). The tablet, which can be swallowed whole or dispersed in water, can be taken with or without food and does not require refrigeration. This formulation allows at-home administration, making Evrysdi the only non-invasive disease-modifying treatment available for SMA patients.
- In February 2025, Roche announced that the U.S. Food and Drug Administration (FDA) approved a New Drug Application (NDA) for Evrysdi (risdiplam) tablets for people living with spinal muscular atrophy (SMA). The 5 mg tablet, which can be swallowed whole or dispersed in water, represents the only non-invasive disease-modifying treatment available for SMA patients.
- In June 2024, Roche announced that the European Commission approved Alecensa (alectinib) monotherapy as adjuvant treatment following tumor resection for adult patients with ALK-positive non-small cell lung cancer (NSCLC) at high risk of recurrence (Stage IB [≥4 cm]–IIIA). The approval was supported by data from the Phase III ALINA trial, which demonstrated a 76% reduction in the risk of disease recurrence or death in patients with resected ALK-positive NSCLC.
- In April 2023, Roche announced that the U.S. Food and Drug Administration (FDA) approved Polivy (polatuzumab vedotin-piiq) in combination with Rituxan (rituximab), cyclophosphamide, doxorubicin, and prednisone (R-CHP) for the treatment of adult patients with previously untreated diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma (HGBL) with an International Prognostic Index (IPI) score of two or greater. This approval converted the prior accelerated approval of Polivy in combination with bendamustine and Rituxan for relapsed or refractory DLBCL into a regular approval, expanding its use in frontline therapy.
Regional Analysis
Geographically, the countries covered in the Dominican Republic High-Cost Medicines market report is the Dominican Republic.
As per Data Bridge Market Research analysis:
For more detailed information about the Dominican Republic High-Cost Medicines market report, click here – https://www.databridgemarketresearch.com/reports/dominican-republic-high-cost-medicines-market