임상시험용 의약품 시장에서는 보관, 제조, 포장, 라벨링 등의 서비스가 매우 중요합니다. 보관 서비스는 다양한 온도 조건에서 임상시험용 의약품을 안전하게 보관하고 제품의 무결성을 보장하는 데 필수적입니다. 제조 서비스는 엄격한 규정을 준수하는 임상시험용 의약품을 생산하는 것을 포함합니다. 포장 및 라벨링 서비스는 안전하고 정확한 유통을 보장하고, 약물 식별 및 사용 설명서를 제공합니다. 이러한 서비스는 임상시험을 지원하고, 제품 안전성, 추적성, 그리고 임상시험 성공에 필수적인 품질 기준 준수를 보장하는 데 필수적입니다.
전체 보고서는 https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market 에서 확인하세요.
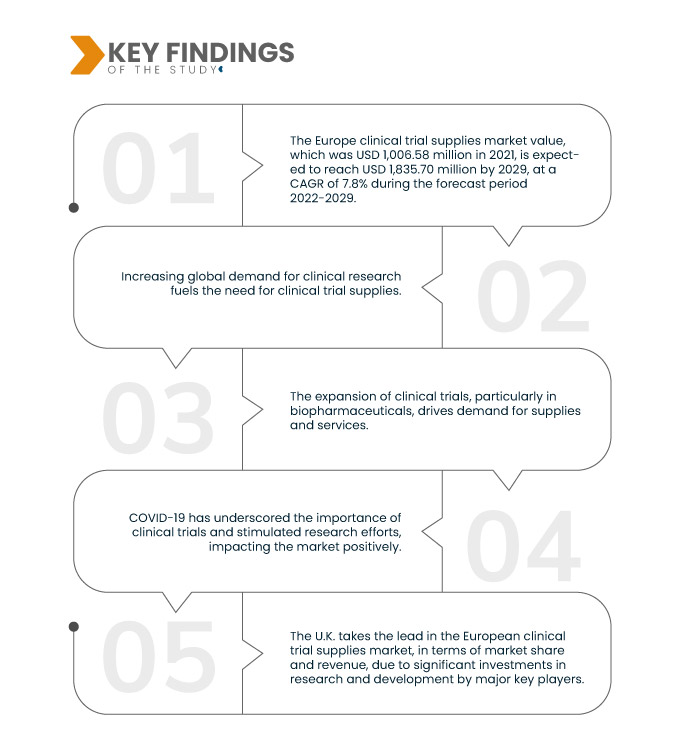
데이터 브리지 마켓 리서치(Data Bridge Market Research)에 따르면, 2021년 10억 658만 달러였던 유럽 임상시험용품 시장 규모는 2029년까지 18억 3,570만 달러로 성장할 것으로 예상되며, 이는 2022년부터 2029년까지 연평균 7.8% 성장한 수치입니다. 임상시험용품 기술 혁신은 효율성과 정확성을 향상시켜 임상시험용품 시장을 주도하고 있습니다. 첨단 도구와 시스템은 공급망 관리를 간소화하고, 오류를 줄이며, 적시 납품을 보장하고, 임상시험의 성공을 지원합니다.
연구의 주요 결과
엄격한 규제 준수로 시장 성장률이 높아질 것으로 예상됩니다.
엄격한 규제 요건은 임상시험 용품 시장의 주요 성장 동력입니다. 규제 기관은 환자 안전과 데이터 무결성을 보장하기 위해 정확하고 규정을 준수하는 임상시험 용품을 요구합니다. 이를 위해서는 꼼꼼한 품질 관리, 추적성 및 문서화가 필수적입니다. 시장은 이러한 엄격한 기준을 충족하는 전문 서비스와 제품을 제공함으로써 이에 대응하고 성장을 촉진합니다. 제약 및 생명공학 기업들은 복잡한 규제 환경을 헤쳐나가고 새로운 치료법을 승인받기 위해 규정을 준수하는 임상시험 용품에 점점 더 의존하고 있습니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2022년부터 2029년까지
|
기준 연도
|
2021
|
역사적인 해
|
2020 (2014-2019년으로 맞춤 설정 가능)
|
양적 단위
|
매출(백만 달러), 볼륨(단위), 가격(달러)
|
다루는 세그먼트
|
서비스(보관, 제조, 포장 및 라벨링), 임상 단계(3상, 2상, 4상, 1상), 치료적 사용(종양학, 심혈관 질환, 피부과, 대사 장애, 감염성 질환 , 호흡기 질환, 중추신경계 및 정신 질환, 혈액 질환, 기타), 최종 사용자별(계약 연구 기관, 제약 및 생명 공학 회사)
|
포함 국가
|
독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 나머지 지역.
|
시장 참여자 포함
|
Movianto(미국), Sharp(미국), Thermo Fisher Scientific Inc.(미국), Catalent, Inc(미국), PCI Pharma Services(미국), Almac Group(영국), PAREXEL International Corporation(미국), Bionical Ltd.(영국), Alium Medical Limited(영국), Myonex(영국), Clinigen Group plc(영국), Ancillare, LP(미국), SIRO Clinpharm(인도), CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC.(미국), Biocair(영국) 등이 있습니다.
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
유럽 임상 시험용품 시장은 서비스, 임상 단계, 치료적 용도, 최종 사용자를 기준으로 분류됩니다.
- 유럽 임상 시험용품 시장은 서비스를 기준으로 제조, 유통, 보관, 포장 및 라벨링으로 구분됩니다.
- 유럽 임상 시험용품 시장은 임상 단계를 기준으로 1상, 2상, 3상, 4상으로 구분됩니다.
- 치료적 용도를 기준으로 유럽 임상시험용품 시장은 종양학, 중추신경계 및 정신 질환, 심혈관 질환, 감염성 질환, 호흡기 질환, 대사 장애, 혈액 질환, 피부과 및 기타로 구분됩니다.
- 최종 사용자를 기준으로 유럽 임상 시험 용품 시장은 계약 연구 기관과 제약 및 생명 공학 회사로 구분됩니다.
주요 플레이어
Data Bridge Market Research에서는 다음 회사를 유럽 임상 시험 용품 시장 주요 기업으로 인식하고 있습니다. 유럽 임상 시험 용품 시장은 Movianto(미국), Sharp(미국), Thermo Fisher Scientific Inc.(미국), Catalent, Inc(미국), PCI Pharma Services(미국), Almac Group(영국), PAREXEL International Corporation(미국)입니다.
시장 개발
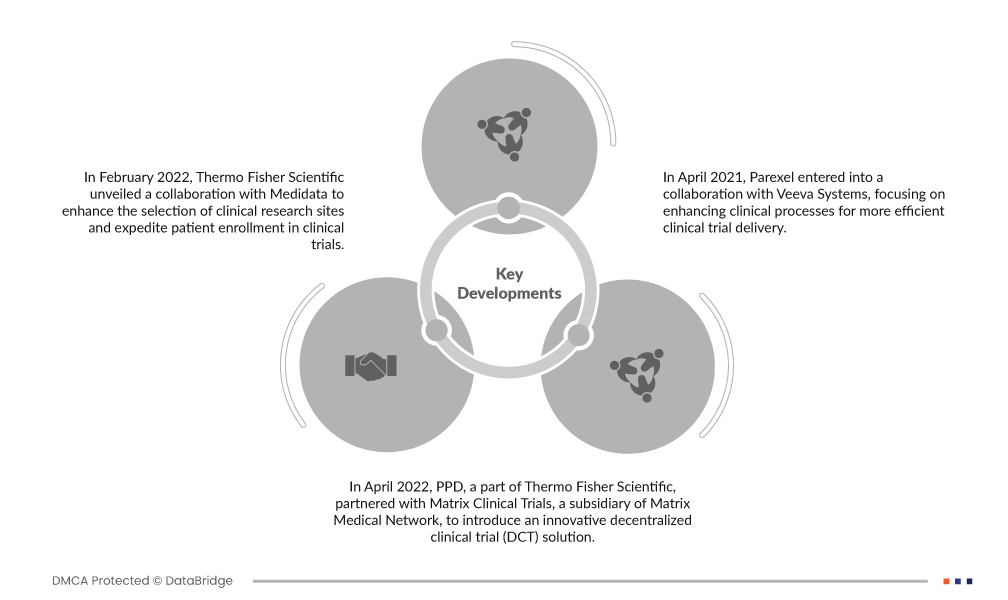
- 2022년 2월, Thermo Fisher Scientific은 Medidata와의 협력을 통해 임상 연구 기관 선정을 개선하고 임상시험 환자 등록을 가속화하는 것을 발표했습니다. 이 협력은 임상시험 계획 및 실행을 간소화하여 궁극적으로 임상시험 진행 속도를 높이는 것을 목표로 합니다. 전 세계 140여 개국에서 수행된 26,000건의 임상시험과 약 800만 명의 환자로부터 수집된 방대한 데이터 세트를 활용하여 더욱 효율적이고 효과적인 임상 연구를 보장합니다.
- 2022년 4월, Thermo Fisher Scientific의 자회사인 PPD는 Matrix Medical Network의 자회사인 Matrix Clinical Trials와 파트너십을 맺고 혁신적인 분산형 임상시험(DCT) 솔루션을 출시했습니다. 이 파트너십은 환자들이 임상시험에 더욱 쉽게 접근할 수 있도록 하는 것을 목표로 합니다. DCT 방식을 통해 환자는 원격으로 임상시험에 참여할 수 있어 편의성과 포용성을 높이는 동시에 고품질 데이터 수집 및 환자 안전 기준을 유지할 수 있습니다.
- 2021년 4월, Parexel은 Veeva Systems와 협력하여 임상 시험의 효율성을 높이기 위한 임상 프로세스 개선에 집중했습니다. 이번 협력은 Veeva의 클라우드 기술을 활용하여 임상 시험의 다양한 측면을 간소화하고 최적화함으로써 연구 개발을 가속화하고, 데이터 관리를 개선하며, 제약 및 생명공학 산업 이해관계자 간의 협업을 강화하는 것을 목표로 합니다.
지역 분석
지리적으로, 유럽 임상 시험 용품 시장 보고서에서 다루는 국가는 독일, 프랑스, 영국, 네덜란드, 스위스, 벨기에, 러시아, 이탈리아, 스페인, 터키, 유럽의 나머지 지역입니다.
Data Bridge Market Research 분석에 따르면:
영국은 2022-2029년 예측 기간 동안 유럽 임상 시험 용품 시장에서 지배적인 지역입니다.
영국은 유럽 임상시험 용품 시장에서 시장 점유율과 매출 면에서 선두를 달리고 있습니다. 이러한 우위는 주요 기업들의 연구개발(R&D)에 대한 막대한 투자로 인해 2022년부터 2029년까지 지속될 것으로 예상됩니다. 이 지역의 선진화된 의료 인프라는 영국이 유럽 시장에서 차지하는 입지를 더욱 강화합니다. 임상시험 용품 서비스 개선에 대한 영국의 지속적인 노력은 영국을 유럽 시장의 핵심 기업으로 자리매김하게 했습니다.
유럽 임상 시험 용품 시장 보고서에 대한 자세한 내용은 여기를 클릭하세요 - https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market