Chronic diseases are non-communicable illnesses that last long and necessitate continuing medical care and management.
According to the World Health Organization, chronic diseases account for nearly 70% of all deaths worldwide, with cardiovascular disease being the primary cause. The rising prevalence of chronic diseases is increasing due to many variables, including an aging population, sedentary lifestyles, poor nutrition, and environmental factors. Radiopharmaceuticals can help healthcare providers to manage chronic diseases more efficiently and effectively.
Different types of chronic diseases include cancer, cardiovascular disease, and diabetes among others which are increasing in Europe. As a result of the sedentary lifestyle of people, the prevalence of lifestyle-associated disorders such as hypertension and diabetes witnessed a rise. Thus, the rising prevalence of chronic diseases with the growing integration of healthcare data with portable devices is expected to demand proper health management, leading to the anticipated increasing demand for radiopharmaceuticals in the Europe market in the forecast period.
Access Full Report @ https://www.databridgemarketresearch.com/reports/europe-radiopharmaceuticals-market
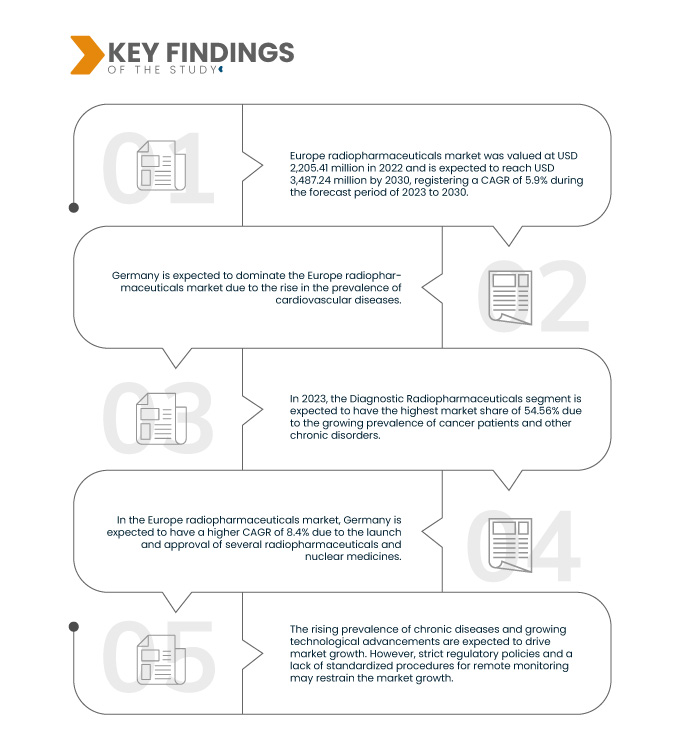
Data Bridge Market Research analyzes that the Europe Radiopharmaceuticals Market is expected to grow with a CAGR of 5.9% from 2023 to 2030 and is expected to reach USD 3,487.24 million by 2030.
Key Findings of the Study
Growing Demand for Radiopharmaceuticals in Diagnostics and Clinical Use
Radiopharmaceuticals have become essential in helping to diagnose organs and treat pathological disorders. They are utilized in diagnostic imaging and radiotherapy. Diagnostic radiopharmaceuticals possess no pharmacological effects and their administration is also not considered to be associated with relevant clinical side effects.
The number of radiopharmaceuticals in clinical use is rapidly growing, thus, allowing the medical community better access to detailed information on the characteristics of the different types of tumors. Radionuclides are also gaining importance by providing palliative and curative treatment for an increasing number of malignant diseases.
Radiopharmaceuticals are used to diagnose complex diseases such as cancer and have been proven efficient in clinical use which increases its demand and is expected to drive market growth.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Year
|
2021 (Customizable to 2015 – 2020)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
Type (Diagnostic Radiopharmaceuticals and Therapeutic Radiopharmaceuticals), Application (Diagnostic and Therapeutic), Source (Nuclear reactors and Cyclotrons), End User (Hospitals, Diagnostic Centers, Cancer research institutes, Ambulatory surgical centers, and Others)
|
|
Countries Covered
|
Germany, U.K., France, Italy, Spain, Belgium, Poland, Russia, Norway, Turkey, Austria, Ireland, Hungary, Netherlands, Switzerland, and Rest of Europe
|
|
Market Players Covered
|
Cardinal Health (U.S.), Advanced Accelerator Applications, A Novartis Company (France), Lantheus (U.S..), CURIUM (France), (U.S..), Jubilant Radiopharma, A Jubilant Pharma Company (India), China lsotope & Radiation Corporation (China), Siemens Healthcare GmbH (Germany), Bracco (U.S..), Eckert & Ziegler (Germany), SHINE Technologies, LLC (U.S..), and IBA Worldwide (Belgium) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, competitive analysis, Brand analysis, technological trends, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The Europe radiopharmaceuticals market is segmented into four notable segments based on type, source, application, and end user.
- On the basis of type, the market is segmented into diagnostic radiopharmaceuticals and therapeutic radiopharmaceuticals.
In 2023, the diagnostic radiopharmaceuticals segment is expected to dominate the Europe radiopharmaceuticals market.
In 2023, the diagnostic radiopharmaceuticals segment is expected to dominate the market with a market share of 54.56%, owing to the increasing prevalence of chronic diseases and the rising adoption of nuclear medicine for treatment and diagnosis by physicians and patients.
- On the basis of application, the market is segmented into diagnostic and therapeutic. In 2023, the diagnostic segment is expected to dominate the market with a market share of 51.91%.
- On the basis of source, the market is segmented into nuclear reactors and cyclotrons. In 2023, the nuclear reactors segment is expected to dominate the market with a market share of 57.91%.
- On the basis of end user, the market is segmented into hospitals, diagnostic centers, cancer research institutes, Ambulatory surgical centers, and others.
In 2023, the hospital segment is expected to dominate the Europe radiopharmaceuticals market.
In 2023, the hospitals segment is expected to dominate the market with a market share of 35.67%, due to the increasing adoption of radiopharmaceuticals for chronic patients for diagnosis and treatment of cancer and CVD patients.
Major Players
Cardinal Health (U.S.), Advanced Accelerator Applications, A Novartis Company (France), Lantheus (U.S..), CURIUM (France), GENERAL ELECTRIC COMPANY (U.S.) among others.
Market Developments
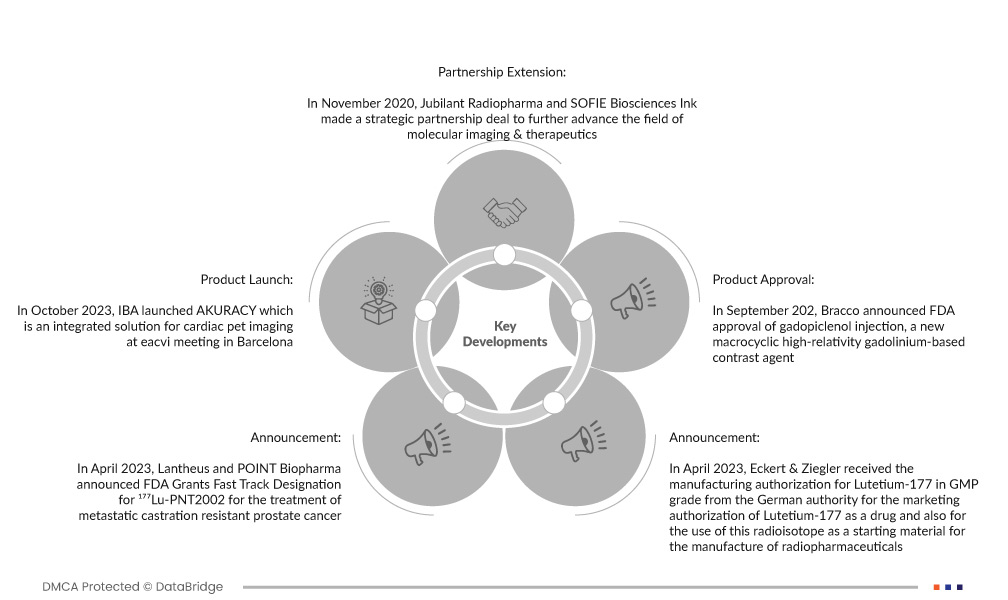
- In October 2023, IBA launched AKURACY which is an integrated solution for cardiac pet imaging at an eacvi meeting in Barcelona. The company believes and states that this is a unique integrated solution for pet myocardial perfusion imaging.
- In April 2023, Lantheus and POINT Biopharma announced the FDA Grants Fast Track Designation for ¹⁷⁷Lu-PNT2002 for the treatment of metastatic castration-resistant prostate cancer. The company stated that the radioligand therapy is quickly becoming another pillar of cancer treatment.
- In April 2023, Eckert & Ziegler received the manufacturing authorization for Lutetium-177 in GMP grade from the German authority for the marketing authorization of Lutetium-177 as a drug and also for the use of this radioisotope as a starting material for the manufacture of radiopharmaceuticals. The company believes this may help in the treatment of neuroendocrine tumors and prostate cancer.
- In December 2022, Novartis received approval for Pluvicto as the first targeted radioligand therapy for the treatment of prostate cancer. The company believes that this will help in advancing a broad portfolio of radioligand therapies to treat cancer in Europe.
- In September 2022 Bracco announced FDA approval of gadopiclenol injection, a new macrocyclic high-relativity gadolinium-based contrast agent. The company believes that this will help to use with MRI in adults and pediatric patients aged 2 years and older to detect and visualize lesions in the central nervous system.
- In November 2020, Jubilant Radiopharma and SOFIE Biosciences Ink made a strategic partnership deal to further advance the field of molecular imaging & therapeutics. The company believes this will help to grow production capacity and will also help in advancing its theranostic pipeline.
Regional Analysis
Geographically, the countries covered in the Europe radiopharmaceuticals market report are Germany, U.K., France, Italy, Spain, Belgium, Poland, Russia, Norway, Turkey, Austria, Ireland, Hungary, Netherlands, Switzerland, and Rest of Europe.
As per Data Bridge Market Research analysis:
Germany is the dominant and fastest country Europe in the market during the forecast period 2023 - 2030.
Germany is expected to dominate and fastest due to the rising adoption of radiopharmaceuticals all across the region. The rising supply of radiopharmaceuticals to patients and the rising practice of monitoring patients for all chronic and non-chronic illnesses are expected to drive market growth in the region.
For more detailed information about the radiopharmaceuticals market, click here – https://www.databridgemarketresearch.com/reports/europe-radiopharmaceuticals-market