Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market is expected to grow with the CAGR of 14.4% in the forecast period of 2021 to 2028. The years considered for study are as mentioned below.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
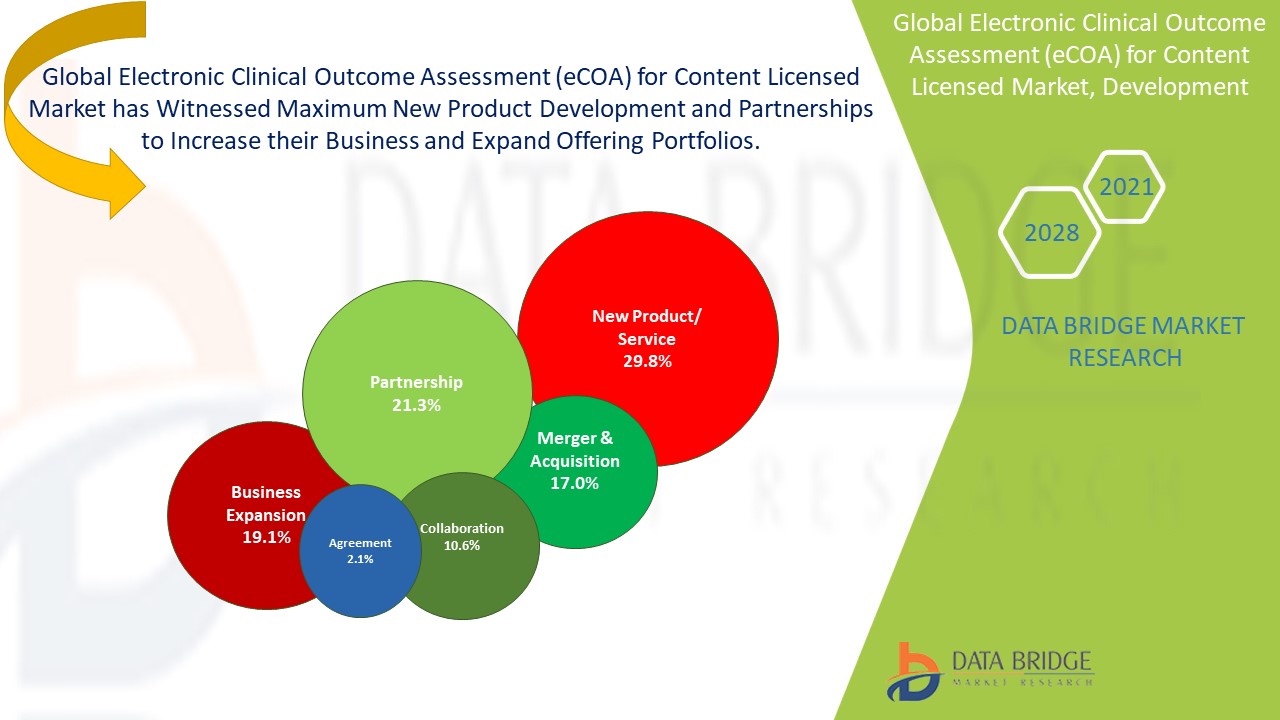
Global electronic clinical outcome assessment (eCOA) for content licensed market is a highly consolidated market, which includes specific number of key players as well as local players. The market has witnessed increased strategic developments owing to favourable market scenario.
The major players dealing in global electronic clinical outcome assessment (eCOA) for content licensed market are introducing strong range of service portfolio. This helped companies to maximize the sales with enhanced product portfolio.
For instance,
- In February 2021, Dassault Systemes’ subsidiary company Medidata has launched Sensor Cloud, a platform to manage a range of sensor and digital health technology data during clinical trials. This technology will allow for remote monitoring in clinical trials of data including vital signs, movement and sleep patterns. With this product launch, company has diversified its product line.
Oracle is the dominating player in global electronic clinical outcome assessment (eCOA) for content licensed market. The other key players existing in the market includes IBM Corporation, Dassault Systemes, Parexel International Corporation, ERT Clinical, eClinical Solutions LLC, ArisGlobal, Clinical Ink, Kayentis, Anju Software, Inc., Signant Health, WIRB-Copernicus Group, YPrime LLC and Bioclinica among others.
Oracle headquartered in Texas, U.S. which was established in 1997. The company is engaged in manufacturing and providing information technology (IT) environments and IT deployment models for enterprises. The company has various business segments including cloud and license, hardware, and services among which cloud and license is the focused business segment. The company offers wide range of product categories such as infrastructure and applications among which applications is a focused category.
- In October 2020, Oracles’ Health Sciences, a division of Oracle specializing in cloud-based life sciences technology is working together with Contract Research organization (CRO) FHI Clinical to create solutions that will boost clinical trial efficiency.
The company has wide global presence across the globe such as Europe, Asia-Pacific, North America, South America and South Africa. In addition to it, the company also generates its revenue from the various subsidiary companies such as NetSuite (U.S.), Taleo (U.S.), Art Technology Group (U.S.), Responsys (U.S.), ORACLE CORPORATION JAPAN (Japan) and others.
Parexel International Corporation
Parexel International Corporation headquartered in North Carolina, U.S. The company is engaged in a suite of biopharmaceutical services of clinical trials to regulatory and consulting. The company offers wide range of product categories parexel biotech, clinical development, consulting, medical affairs, real-world affairs and fsp, regulatory & access consulting among which clinical development, regulatory & access consulting is market focused category.
- In September 2020, Parexel International Corporation, a leading provider of solutions for clinical research and medical therapies has launched a new Regulatory Submissions Hub Service for life science and biopharmaceutical customers in response to the impending implementation of EU Clinical Trial Regulation 536/2014. With this new launch, company will be able to offer the customized solutions to manage every aspect of their customer.
The company has presence in North America, Asia, Europe, South America, Middle East and Africa.
ERT Clinical
ERT Clinical is headquartered in Pennsylvania, U.S. which was founded in 1972. The company is engaged in providing solutions for clinical trials and therapeutic applications. The company has product categories which are safety & efficacy data, data analytics, real world evidence, expert solutions and virtual capabilities in which safety & efficacy data is market focused category.
- In February 2020, ERT Clinical, a leading global data and technology company for clinical endpoint data collection has introduced a powerful new solution eCOA Multimedia for enabling the collection, processing and analysis of photos and audio as part of clinical trial eCOA assessments. With this new launch, company has increased its product line.
The company has global presence in North America, Europe and Asia.