The increasing demand for regulatory affairs outsourcing services has significantly boomed worldwide due to the growing demand for medical devices. Strategic initiatives such as acquisition, partnership, and contract agreement among others provide many opportunities to flourish their customer base geographically. The increasing number of clinical trials globally is being boosted by the increasing demand for new medical equipment and therapies among various end users and growing investment in R&D activities for developing effective medicines.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-medical-device-regulatory-affairs-outsourcing-market
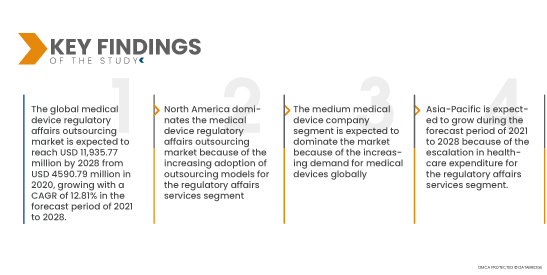
Data Bridge Market Research analyses that the Global Medical Device Regulatory Affairs Outsourcing Market is expected to reach USD 11,935.77 million by 2028 from USD 4590.79 million in 2020, growing with a CAGR of 12.81% in the forecast period of 2021 to 2028. The increasing demand for the medical device regulatory affairs outsourcing across different sectors will provide potential market growth opportunities in the forecast period.
The increasing number of clinical trials is anticipated to drive the market's growth rate
The growing number of clinical trials globally is driven by growing demand for new medical equipment and therapies among different end users and rising investment in R&D activities for developing effective medicines. Additionally, a rise in the number of people suffering from chronic diseases along with improvements in the circumstances and nature of some forms of chronic diseases is expected to boost major growth in clinical trials all over the world. Furthermore, the incidence of neurological disorders such as Alzheimer's, cancers, Parkinson's, depression and epilepsy as well as an ageing population is boosting the number of clinical trials.
For instance,
As per the records of the ClinicalTrials.gov Results Database, as of 5th April, 2021, total number of registered studies worldwide was found to be 373,420. Among them, 51% studies were the non-U.S. based and 33% clinical trial studies were the U.S. based only
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2021 to 2028
|
|
Base Year
|
2020
|
|
Historic Years
|
2019 (Customizable to 2013 - 2018)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Services (Regulatory Affairs Services, Quality Consulting and Medical Writing), Product (Finished Goods, Electronics and Raw Material), Device Type (Class I, Class II and Class III), Application (Cardiology, Diagnostic Imaging, Orthopedic, IVD, Ophthalmic, General and Plastic Surgery, Drug Delivery, Dental, Endoscopy, Diabetes Care and Others), End User (Small Medical Device Company, Medium Medical Device Company and Large Medical Device Company)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Ireland, rest of Europe, Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America, Saudi Arabia, South Africa, UAE, Israel, Egypt and rest of Middle East and Africa
|
|
Market Players Covered
|
Parexel International Corporation (U.S.), North American Science Associates, Inc. (U.S.), SGS SA (Switzerland), Pace Analytical Services, LLC (U.S.), Trilogy Writing & Consulting GmbH (Germany), Creganna (Galway), American Preclinical Services, LLC (U.S.), Intertek Group plc (U.K.), WuXi AppTec (China), Charles River Laboratories (U.S.), Celestica Inc. (Canada), Freyr (U.S.), Cactus Communications (India), Cekindo Business International (Indonesia), Eurofins Scientific (Luxembourg), Covance (U.S.), Plexus Corp. (U.S.), Sanmina Corporation (U.S.), Omron Corporation (Japan)
|
|
Data Points Covered in the Report
|
In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
Global medical device regulatory affairs outsourcing market is categorized into five notable segments which are based on the services, product, device type, application and end user.
- On the basis of services, global medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting and medical writing.
The regulatory affairs services segment of services type is anticipated to dominate the medical device regulatory affairs outsourcing market
The regulatory affairs services segment is expected to dominate the market with 62.19% market share due to the increased adoption of regulatory affairs outsourcing by major medical device companies.
- On the basis of product, global medical device regulatory affairs outsourcing market is segmented into finished goods, electronics and raw material.
The finished goods segment of product type is anticipated to dominate the medical device regulatory affairs outsourcing market
The finished goods segment is expected to dominate the market with 44.64% market share because of the increased adoption of regulatory affairs outsourcing for the finished goods by major medical device companies.
- On the basis of device type, global medical device regulatory affairs outsourcing market is segmented into class I, class II and class III. The class I segment is expected to dominate the market with 41.57% market share because of the increasing demand for medical devices globally to treat patients with chronic diseases.
- On the basis of application, global medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care and others. The cardiology segment is expected to dominate the market with 20.50% market share due to the increased adoption of regulatory affairs outsourcing for the class III medical devices by major medical device market players.
- On the basis of end user, global medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company and large medical device company. The medium medical device company segment is expected to dominate the market with 47.25% market share because of the increasing demand for medical devices globally.
Major Players
Data Bridge Market Research recognizes the following companies as the major medical device regulatory affairs outsourcing market players in medical device regulatory affairs outsourcing market are Parexel International Corporation (U.S.), North American Science Associates, Inc. (U.S.), SGS SA (Switzerland), Pace Analytical Services, LLC (U.S.), Trilogy Writing & Consulting GmbH (Germany), Creganna (Galway), American Preclinical Services, LLC (U.S.), Intertek Group plc (U.K.), WuXi AppTec (China), Charles River Laboratories (U.S.),, Celestica Inc. (Canada), Freyr (U.S.), Cactus Communications (India), Cekindo Business International (Indonesia), Eurofins Scientific (Luxembourg), Covance (U.S.), Plexus Corp. (U.S.), Sanmina Corporation (U.S.), Omron Corporation (Japan)
Market Development
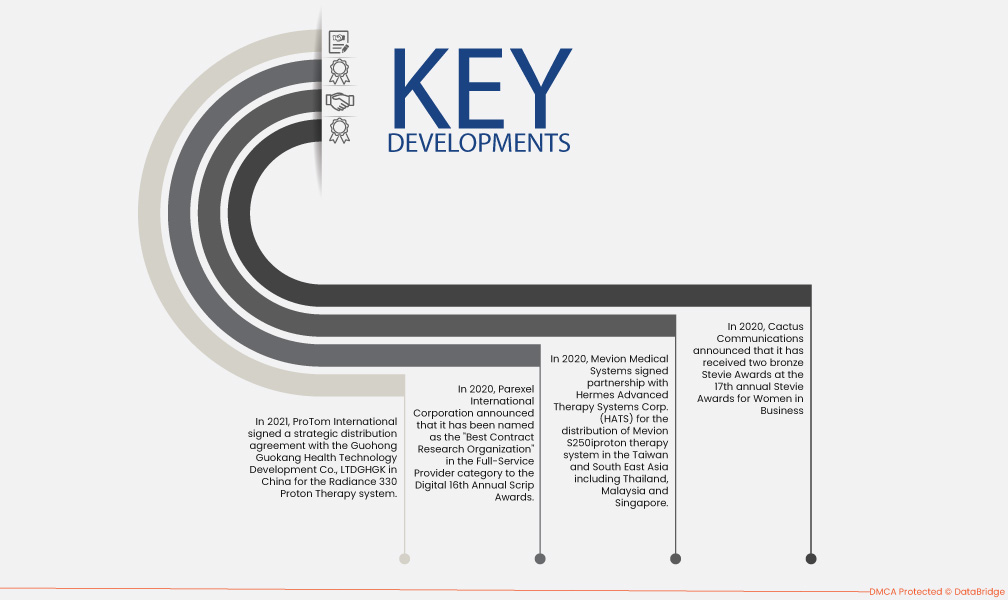
- In 2021, ProTom International signed a strategic distribution agreement with the Guohong Guokang Health Technology Development Co., LTDGHGK in China for the Radiance 330 Proton Therapy system. This agreement is based on the distribution of the product in China Mainland. This agreement specifies the company's international expansion as the global provider of the product.
- In 2020, Mevion Medical Systems signed a partnership with Hermes Advanced Therapy Systems Corp. (HATS) to distribute Mevion S250iproton therapy system in the Taiwan and South East Asia including Thailand, Malaysia and Singapore. This will enhance the business for the company in these regions, making a huge profit of the company, and help in further business expansion.
- In 2020, Parexel International Corporation announced that it has been named as the "Best Contract Research Organization" in the Full-Service Provider category to the Digital 16th Annual Scrip Awards. This recognition received by the company has increased its sales and demand in the market.
- In 2020, Cactus Communications announced that it received two bronze Stevie Awards at the 17th annual Stevie Awards for Women in Business. This recognition received by the company has surged its credibility in the market which leads to increased sales and revenue in future
Regional Analysis
Geographically, the countries covered in the medical device regulatory affairs outsourcing market report are U.S., Canada, Mexico, Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Ireland, rest of Europe, Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America, Saudi Arabia, South Africa, UAE, Israel, Egypt and rest of Middle East and Africa
As per Data Bridge Market Research analysis:
North America is the dominant region in medical device regulatory affairs outsourcing market during the forecast period 2021 to 2028
North America dominates the medical device regulatory affairs outsourcing market because of the increasing number of major market players. Furthermore, the growing adoption of outsourcing models for the regulatory affairs services segment.
Asia-Pacific is estimated to be the fastest-growing region in medical device regulatory affairs outsourcing market the forecast period 2021 to 2028
Asia-Pacific is expected to grow from 2021 to 2028 because of the growing R&D activities for this region's regulatory affairs services segment. In addition to this, growing adoption of advanced technology is also anticipated to propel the market's growth rate in this region.
For more detailed information about medical device regulatory affairs outsourcing market report, click here – https://www.databridgemarketresearch.com/reports/global-medical-device-regulatory-affairs-outsourcing-market