Global Diagnostic Reagents Market
Market Size in USD Billion
CAGR :
% 
 USD
48.90 Billion
USD
84.01 Billion
2022
2030
USD
48.90 Billion
USD
84.01 Billion
2022
2030
| 2023 –2030 | |
| USD 48.90 Billion | |
| USD 84.01 Billion | |
|
|
|
|
Diagnostic Reagents Market Analysis and Size
The market for diagnostic reagents is expected to grow during the forecast period of 2023 to 2030. Diagnostic reagents are lab chemicals to identify particular pathogens, metabolic irregularities, physiological anomalies, and hereditary illnesses. They are employed in vivo or in vitro to identify certain disorders and rely on medical professionals to make reliable diagnosis.
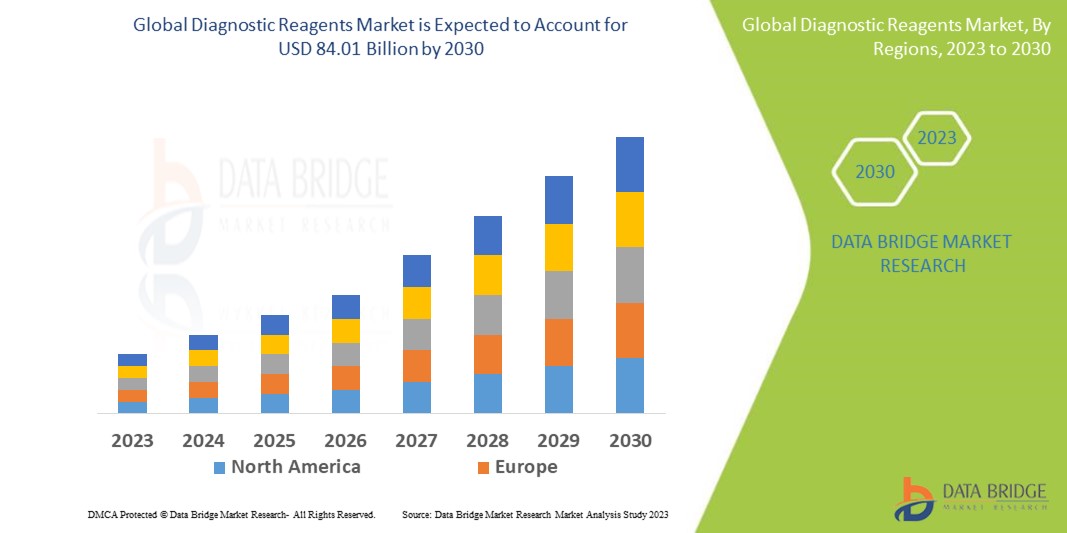
Data Bridge Market Research analyses that the diagnostic reagents market, valued at USD 48.90 billion in 2022, is expected to reach USD 84.01 billion by 2030, growing at a CAGR of 7.00% during the forecast period of 2023 to 2030. “Hospitals” dominates the end segment of the diagnostic reagents market owing to the higher in-house diagnostic testing comparatively to the others in the segment. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Diagnostic Reagents Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Sample Preparation Kits, Microarray Kits, PCR Assay Kits, In Situ Hybridization Kits, Sequencing Kits), End User (Hospitals, Research Institutes, Laboratories, Biopharmaceutical Companies, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Siemens (Germany),Hologic, Inc. (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), Abbott (U.S.), BD (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Koninklijke Philips N.V. (Netherlands), NeuroLogica Corp.(U.S.), Shimadzu Medical (India) pvt. Ltd. (Japan), GENERAL ELECTRIC (U.S.), Quest Diagnostics Incorporated (U.S.), Sysmex India Pvt. Ltd. (Japan), Hitachi, Ltd. (Japan), Canon Inc. (Japan), FUJIFILM Holdings Corporation (U.K.) |
|
Market Opportunities |
|
Market Definition
A diagnostic reagent is any reagent used in vitro or in vivo to detect or screen a particular disease or any health-related issue. Any organic or inorganic substances that are added to an analyte or biological specimen, such as urine, blood, or biopsied human tissue, to identify aetiology or abnormalities are referred to as diagnostic reagents. Substances or compounds used in diagnostic testing to identify, detect, or measure specific biological or chemical substances in patient samples. These reagents are essential components of diagnostic tests and play a crucial role in disease diagnosis, monitoring, and treatment.
Global Diagnostic Reagents Market Dynamics
Drivers
- Increasing Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases is a significant driver for the diagnostic reagents market. Chronic diseases, such as cardiovascular disorders, diabetes, and cancer, have become increasingly common worldwide. Diagnostic reagents play a crucial role in accurately identifying and monitoring these diseases. They are used in various diagnostic procedures, including blood tests, imaging techniques, and genetic testing, to detect biomarkers, specific molecules, or genetic variations associated with these diseases. By enabling early disease detection and monitoring, diagnostic reagents facilitate timely intervention, treatment planning, and disease management. With the rising burden of chronic diseases, the demand for diagnostic reagents continues to grow, driving market expansion.
- Technological Advancements in Diagnostics
Technological advancements in diagnostics have revolutionized the field of medicine and significantly impacted the demand for diagnostic reagents. Molecular diagnostics, immunoassays, and point-of-care testing are among the key technological innovations that have driven the market growth. Molecular diagnostics, including techniques like polymerase chain reaction (PCR) and next-generation sequencing (NGS), have enhanced the sensitivity and specificity of diagnostic tests. These advancements enable the detection of genetic variations, mutations, and infectious agents with high accuracy and precision. Immunoassays, such as enzyme-linked immunosorbent assays (ELISA), utilize specific antibodies to identify and quantify target molecules in patient samples. Point-of-care testing has also gained prominence due to its convenience and rapid results. These technological advancements have expanded the applications of diagnostic reagents, improved diagnostic accuracy, and accelerated testing workflows, leading to increased adoption of diagnostic reagents in healthcare settings.
- Growing Aging Population
The global aging population is a significant driver for the global diagnostic reagents market. As people age, the incidence of age-related diseases and conditions increases. Elderly individuals are more susceptible to chronic diseases, including cardiovascular disorders, neurodegenerative diseases, and certain types of cancer. Regular diagnostic testing becomes essential for disease detection, monitoring, and management in this population. Diagnostic reagents are critical in facilitating early disease detection, identifying disease progression, and monitoring treatment efficacy. They enable healthcare professionals to assess biomarkers, measure specific molecules, and detect genetic variations associated with age-related diseases. The growing aging population, coupled with the increasing focus on preventive healthcare, has led to a higher demand for diagnostic reagents to support geriatric care and improve patient outcomes.
Opportunities
- Emerging Markets
Developing economies present significant growth opportunities for the global diagnostic reagents market. The expanding healthcare infrastructure, increasing access to diagnostic services, and rising awareness about early disease detection in these regions are driving the demand for diagnostic reagents.
- High Prevalence and increase in the Incidence of Chronic Infectious Diseases
One of the most common uses of in vitro diagnostic reagents is the diagnosis of infectious and chronic disorders. Any diagnostic test requires the use of diagnostic reagents, including biological and chemical reagents. IVD reagents are essential in developing new diagnostic tools and tests for the early diagnosis and prevention of diseases, which have become more difficult due to the emergence and breakout of several infectious diseases.
Restraints
- Stringent Regulatory Frameworks
The global diagnostic reagents market faces challenges due to strict regulations imposed by regulatory authorities. The complex approval processes, stringent quality standards, and compliance requirements can hinder market growth and increase the time and cost of product development.
Challenges
- Supply Chain Disruptions
The global diagnostic reagents market is susceptible to supply chain disruptions, such as raw material shortages, transportation issues, and production delays. These disruptions can impact the availability and delivery of diagnostic reagents, leading to challenges in meeting the growing demand and potentially affecting patient care.
This global diagnostic reagents market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global diagnostic reagents market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2021, CYTENA launched C. LIVE Tox, reagents for real-time direct detection of cytotoxicity. C.LIVE Tox Green and Red are sensitive DNA-binding dyes that are nonpermeable to viable cells and nonfluorescent on the outside
- In March 2019, Ortho Clinical Diagnostics launched four new key assays to meet the demand of the rapidly rising diagnostics market in China. In order to address the need of the rapidly expanding diagnostics market, Ortho Clinical Diagnostics, a global pioneer in in vitro diagnostics, has launched four new critical assays. Cystatin C, -Hydroxybutyrate Dehydrogenase (HBDH), Homocysteine (HCY), and Total Bile Acid (TBA) are the assays that are added as part of this product launch, which was made possible by a partnership with Beijing Leadman Biochemistry Co., Ltd. of Beijing
Global Diagnostic Reagents Market Scope
The global diagnostic reagents market is segmented on the basis of product type and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Chromatography Reagents
- Molecular Diagnostic Reagents
- Immunoassay Reagents
- Clinical Chemistry Reagents
- Flow Cytometry Reagents
- Cell and Tissue Culture Reagents
- Hematology and Hemostasis Reagents
- Microbiology Reagents
- Others
End-User
- Hospitals
- Research Institutes
- Laboratories
- Biopharmaceutical Companies
- Others
Diagnostic Reagents Market Regional Analysis/Insights
The diagnostic reagents market is analysed and market size insights and trends are provided by country, product type and end-user as referenced above.
The countries covered in the diagnostic reagents market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the global diagnostic reagents market due to the increasing use of molecular diagnostics in cancer screening and genetic disorders availability of government funds and the presence of major key players in the region.
Asia-Pacific is expected to grow at the highest growth rate in the global diagnostic reagents market in the forecast period of 2029 to 2030 due to the increase in popularity of molecular test over traditional tests such as microbiology test, surge in the incidence of lifestyle-related and chronic illnesses.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The diagnostic reagents market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for diagnostic reagents market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the diagnostic reagents market. The data is available for historic period 2015-2020.
Competitive Landscape and Diagnostic Reagents Market Share Analysis
The diagnostic reagents market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global diagnostic reagents market.
Some of the major players operating in the global diagnostic reagents market are:
- Siemens (Germany)
- Hologic, Inc. (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Abbott (U.S.)
- BD (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Thermo Fisher Scientific Inc. (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- NeuroLogica Corp.(U.S.)
- Shimadzu Medical (India) pvt. Ltd. (Japan)
- GENERAL ELECTRIC (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Sysmex India Pvt. Ltd. (Japan)
- Hitachi, Ltd. (Japan)
- Canon Inc. (Japan)
- FUJIFILM Holdings Corporation (U.K.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL DIAGNOSTIC REAGENTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL DIAGNOSTIC REAGENTS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL DIAGNOSTIC REAGENTS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY TYPE
16.1 OVERVIEW
16.2 CHROMATOGRAPHY REAGENTS
16.3 MOLECULAR DIAGNOSTIC REAGENTS
16.4 IMMUNOASSAY REAGENTS
16.5 CLINICAL CHEMISTRY REAGENTS
16.6 FLOW CYTOMETRY REAGENTS
16.7 CELL AND TISSUE CULTURE REAGENTS
16.8 HEMATOLOGY AND HEMOSTASIS REAGENTS
16.9 MICROBIOLOGY REAGENTS
16.1 OTHERS
17 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY PRODUCTS
17.1 OVERVIEW
17.2 MOLECULAR DIAGNOSTIC REAGENTS
17.2.1 NUCLEIC ACID EXTRACTION REAGENTS
17.2.1.1. DNA EXTRACTION REAGENTS
17.2.1.2. RNA EXTRACTION REAGENTS
17.2.2 POLYMERASE CHAIN REACTION (PCR) REAGENTS
17.2.2.1. DNA POLYMERASE
17.2.2.2. PRIMERS
17.2.2.3. NUCLEOTIDE MIX
17.2.2.4. BUFFER SOLUTIONS
17.2.2.5. MGCL2
17.2.2.6. OTHERS
17.2.3 REVERSE TRANSCRIPTION REAGENTS
17.2.3.1. REVERSE TRANSCRIPTASE
17.2.3.2. RANDOM PRIMERS OR OLIGO(DT)
17.2.4 QUANTITATIVE PCR (QPCR) REAGENTS
17.2.4.1. FLUORESCENT PROBES
17.2.4.2. REFERENCE STANDARDS
17.2.4.3. OTHERS
17.2.5 DNA/RNA LABELING AND DETECTION
17.2.5.1. FLUORESCENT DYES OR RADIOACTIVE PROBES
17.2.5.2. CHEMILUMINESCENT SUBSTRATES
17.2.5.3. OTHERS
17.2.6 NEXT-GENERATION SEQUENCING (NGS) REAGENTS
17.2.6.1. SEQUENCING REAGENTS
17.2.6.2. INDEXING PRIMERS
17.2.6.3. OTHERS
17.2.7 PROTEIN DETECTION REAGENTS
17.2.8 ISOTHERMAL AMPLIFICATION REAGENTS
17.2.9 CRISPR-CAS REAGENTS
17.2.9.1. GUIDE RNA (GRNA)
17.2.9.2. CAS PROTEIN
17.2.9.3. OTHERS
17.2.10 HYBRIDIZATION AND MICROARRAY REAGENTS
17.2.10.1. CAPTURE PROBES
17.2.10.2. DETECTION PROBES
17.2.10.3. OTHERS
17.3 IMMUNOASSAY REAGENTS
17.3.1 ANTIBODIES
17.3.1.1. PRIMARY ANTIBODIES
17.3.1.2. SECONDARY ANTIBODIES
17.3.2 ENZYMES
17.3.2.1. HORSERADISH PEROXIDASE (HRP)
17.3.2.2. ALKALINE PHOSPHATASE (AP)
17.3.3 FLUOROPHORES
17.3.3.1. FLUORESCEIN ISOTHIOCYANATE (FITC)
17.3.3.2. RHODAMINE
17.3.3.3. ALEXA FLUOR DYES
17.3.3.4. OTHERS
17.3.4 CHEMILUMINESCENT SUBSTRATES
17.3.5 BIOTINYLATION REAGENTS
17.3.6 BLOCKING REAGENTS
17.3.7 CALIBRATORS AND CONTROLS
17.3.7.1. CALIBRATION STANDARDS
17.3.7.2. POSITIVE AND NEGATIVE CONTROLS
17.3.8 BUFFER SOLUTIONS
17.3.8.1. PBS (PHOSPHATE-BUFFERED SALINE)
17.3.8.2. TRIS-HCL
17.3.8.3. OTHERS
17.3.9 RECOMBINANT OR PURIFIED ANTIGENS
17.3.10 WASH BUFFERS
17.3.11 OTHERS
17.4 CLINICAL CHEMISTRY REAGENTS
17.4.1 ENZYMES
17.4.1.1. ALANINE AMINOTRANSFERASE (ALT) REAGENTS
17.4.1.2. ASPARTATE AMINOTRANSFERASE (AST) REAGENTS
17.4.1.3. ALKALINE PHOSPHATASE (ALP) REAGENTS
17.4.1.4. OTHERS
17.4.2 SUBSTRATES
17.4.2.1. GLUCOSE REAGENTS
17.4.2.2. OTHERS
17.4.3 LIPID PROFILE REAGENTS
17.4.3.1. CHOLESTEROL REAGENTS
17.4.3.2. TRIGLYCERIDE REAGENTS
17.4.3.3. HIGH-DENSITY LIPOPROTEIN (HDL) CHOLESTEROL REAGENTS
17.4.3.4. LOW-DENSITY LIPOPROTEIN (LDL) CHOLESTEROL REAGENTS
17.4.4 ELECTROLYTES
17.4.4.1. SODIUM
17.4.4.2. POTASSIUM
17.4.4.3. CHLORIDE REAGENTS
17.4.4.4. OTHERS
17.4.5 KIDNEY FUNCTION REAGENTS
17.4.5.1. BLOOD UREA NITROGEN (BUN) REAGENTS
17.4.5.2. CREATININE REAGENTS
17.4.5.3. OTHER
17.4.6 LIVER FUNCTION REAGENTS
17.4.6.1. BILIRUBIN REAGENTS
17.4.6.2. GAMMA-GLUTAMYL TRANSFERASE (GGT) REAGENTS
17.4.6.3. OTHERS
17.4.7 CARDIAC MARKERS
17.4.7.1. TROPONIN REAGENTS
17.4.7.2. CREATINE KINASE (CK) REAGENTS
17.4.7.3. OTHERS
17.4.8 HORMONES
17.4.8.1. THYROID FUNCTION REAGENTS
17.4.8.2. CORTISOL REAGENTS
17.4.8.3. OTHERS
17.4.9 PROTEINS
17.4.9.1. ALBUMIN REAGENTS
17.4.9.2. TOTAL PROTEIN REAGENTS
17.4.9.3. OTHERS
17.4.10 COAGULATION REAGENTS
17.4.10.1. PROTHROMBIN TIME (PT) REAGENTS
17.4.10.2. ACTIVATED PARTIAL THROMBOPLASTIN TIME (APTT) REAGENTS
17.4.10.3. OTHERS
17.4.11 URINE CHEMISTRY REAGENTS
17.4.11.1. URINE GLUCOSE REAGENTS
17.4.11.2. URINE PROTEIN REAGENTS
17.4.11.3. OTHERS
17.4.12 SPECIFIC PROTEINS
17.4.12.1. C-REACTIVE PROTEIN (CRP) REAGENTS
17.4.12.2. HEMOGLOBIN A1C (HBA1C) REAGENTS
17.4.12.3. OTHERS
17.5 FLOW CYTOMETRY REAGENTS
17.5.1 FLUOROCHROME-CONJUGATED ANTIBODIES
17.5.1.1. FLUORESCEIN ISOTHIOCYANATE (FITC)
17.5.1.2. PHYCOERYTHRIN (PE)
17.5.1.3. PERIDININ-CHLOROPHYLL PROTEIN (PERCP)
17.5.1.4. ALLOPHYCOCYANIN (APC)
17.5.1.5. ALEXA FLUOR DYES
17.5.1.6. OTHERS
17.5.2 CELL VIABILITY DYES
17.5.2.1. PROPIDIUM IODIDE (PI)
17.5.2.2. 7-AMINOACTINOMYCIN D (7-AAD)
17.5.2.3. FIXABLE VIABILITY DYES
17.5.2.4. OTHERS
17.5.3 NUCLEIC ACID STAINS
17.5.3.1. 4',6-DIAMIDINO-2-PHENYLINDOLE (DAPI)
17.5.3.2. HOECHST DYES
17.5.3.3. OTHERS
17.5.4 ISOTYPE CONTROLS
17.5.5 OTHERS
17.6 CELL AND TISSUE CULTURE REAGENTS
17.6.1 CELL CULTURE MEDIA
17.6.1.1. DULBECCO'S MODIFIED EAGLE MEDIUM (DMEM)
17.6.1.2. RPMI 1640
17.6.1.3. MINIMUM ESSENTIAL MEDIUM (MEM)
17.6.1.4. OTHERS
17.6.2 SERUM
17.6.2.1. FETAL BOVINE SERUM (FBS)
17.6.2.2. BOVINE SERUM ALBUMIN (BSA)
17.6.2.3. OTHERS
17.6.3 ANTIBIOTICS AND ANTIMYCOTICS
17.6.3.1. PENICILLIN-STREPTOMYCIN
17.6.3.2. AMPHOTERICIN B
17.6.3.3. OTHERS
17.6.4 TRYPSIN AND TRYPSIN INHIBITORS
17.6.4.1. TRYPSIN
17.6.4.2. TRYPSIN-EDTA
17.6.4.3. TRYPSIN INHIBITOR
17.6.4.4. OTHERS
17.6.5 CELL DISSOCIATION REAGENTS
17.6.5.1. ACCUTASE
17.6.5.2. COLLAGENASE
17.6.5.3. DISPASE
17.6.5.4. OTHERS
17.6.6 GROWTH FACTORS AND CYTOKINES
17.6.7 CELL FREEZING AND THAWING REAGENTS
17.6.7.1. DMSO (DIMETHYL SULFOXIDE)
17.6.7.2. CRYOPROTECTIVE AGENTS
17.6.7.3. OTHERS
17.6.8 EXTRACELLULAR MATRIX PROTEINS
17.6.8.1. COLLAGEN
17.6.8.2. FIBRONECTIN
17.6.8.3. LAMININ
17.6.8.4. OTHERS
17.6.9 HYBRIDOMA CULTURE REAGENTS
17.6.10 OTHERS
17.7 HEMATOLOGY AND HEMOSTASIS REAGENTS
17.7.1 HEMATOLOGY REAGENTS
17.7.1.1. HEMATOLOGY STAINS
17.7.1.1.1. WRIGHT-GIEMSA STAIN
17.7.1.1.2. NEW METHYLENE BLUE STAIN
17.7.1.2. CBC REAGENTS
17.7.1.2.1. DILUENT
17.7.1.2.2. LYSING REAGENTS
17.7.1.2.3. OTHERS
17.7.1.3. AUTOMATED HEMATOLOGY ANALYZER REAGENTS
17.7.1.3.1. HEMATOLOGY CONTROLS
17.7.1.3.2. CALIBRATION STANDARDS
17.7.1.3.3. OTHERS
17.7.1.4. MANUAL CELL COUNTING REAGENTS
17.7.1.5. HEMOGLOBIN REAGENTS
17.7.1.6. ERYTHROCYTE SEDIMENTATION RATE (ESR) REAGENTS
17.7.1.7. OTHERS
17.7.2 HEMOSTASIS REAGENTS
17.7.2.1. PROTHROMBIN TIME (PT) REAGENTS
17.7.2.2. THROMBOPLASTIN REAGENTS
17.7.2.3. ACTIVATED PARTIAL THROMBOPLASTIN TIME (APTT) REAGENTS
17.7.2.4. CLAUSS METHOD REAGENTS
17.7.2.5. ANTICOAGULANT MONITORING REAGENTS
17.7.2.6. OTHERS
17.8 MICROBIOLOGY REAGENTS
17.8.1 BIOCHEMICAL TEST REAGENTS
17.8.1.1. TRIPLE SUGAR IRON (TSI)
17.8.1.2. SIMMONS' CITRATE AGAR
17.8.1.3. UREA AGAR
17.8.1.4. OTHERS
17.8.2 SEROLOGICAL REAGENTS
17.8.2.1. ANTIBODY REAGENTS
17.8.2.2. ANTIGEN DETECTION REAGENTS
17.8.2.3. OTHERS
17.8.3 ANTIMICROBIAL SUSCEPTIBILITY TESTING (AST) REAGENTS
17.8.4 MYCOLOGY REAGENTS
17.8.4.1. SABOURAUD DEXTROSE AGAR (SDA)
17.8.4.2. LACTOPHENOL COTTON BLUE STAIN
17.8.4.3. OTHERS
17.8.5 MICROBIAL PRESERVATION REAGENTS
17.8.6 LYOPHILIZATION REAGENTS
17.8.7 OTHERS
17.9 CHROMATOGRAPHY REAGENTS
17.1 OTHERS
18 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY NATURE
18.1 OVERVIEW
18.2 CHEMICAL REAGENTS
18.3 BIOLOGICAL REAGENTS
19 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 INFECTIOUS DISEASE TESTING
19.2.1 HIV
19.2.2 HEPATITIS
19.2.3 TUBERCULOSIS
19.2.4 COVID-19
19.2.5 OTHERS
19.3 ONCOLOGY
19.3.1 CANCER BIOMARKERS
19.3.2 GENETIC TESTING
19.3.3 OTHERS
19.4 CARDIOLOGY
19.4.1 CARDIAC MARKERS
19.4.2 LIPID PROFILES
19.4.3 OTHERS
19.5 DIABETES:
19.5.1 GLUCOSE TESTING
19.5.2 HBA1C TESTING
19.5.3 OTHERS
19.6 HEMATOLOGY
19.6.1 COMPLETE BLOOD COUNT (CBC)
19.6.2 COAGULATION TESTS
19.6.3 OTHERS
19.7 OTHERS
20 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 DIAGNOSTIC CENTER
20.4 RESEARCH LABS & INSTITUTES
20.5 BLOOD BANKS
20.6 SPECILATY CLINICS
20.7 AMBULATORY SURGICAL CENTERS (ASCS)
20.8 OTHERS
21 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDERS
21.3 RETAIL SALES
21.4 ONLINE SALES
21.5 OTHERS
22 GLOBAL DIAGNOSTIC REAGENTS MARKET, COMPANY LANDSCAPE
22.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
22.2 COMPANY SHARE ANALYSIS: EUROPE
22.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
22.4 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
22.5 COMPANY SHARE ANALYSIS: SOUTH AMERICA
22.6 MERGERS & ACQUISITIONS
22.7 NEW PRODUCT DEVELOPMENT & APPROVALS
22.8 EXPANSIONS
22.9 REGULATORY CHANGES
22.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
23 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY REGION
GLOBAL DIAGNOSTIC REAGENTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 NORTH AMERICA
23.1.1 U.S.
23.1.2 CANADA
23.1.3 MEXICO
23.2 EUROPE
23.2.1 GERMANY
23.2.2 FRANCE
23.2.3 U.K.
23.2.4 HUNGARY
23.2.5 LITHUANIA
23.2.6 AUSTRIA
23.2.7 IRELAND
23.2.8 NORWAY
23.2.9 POLAND
23.2.10 ITALY
23.2.11 SPAIN
23.2.12 RUSSIA
23.2.13 TURKEY
23.2.14 BELGIUM
23.2.15 NETHERLANDS
23.2.16 SWITZERLAND
23.2.17 REST OF EUROPE
23.3 ASIA-PACIFIC
23.3.1 JAPAN
23.3.2 CHINA
23.3.3 SOUTH KOREA
23.3.4 INDIA
23.3.5 AUSTRALIA
23.3.6 SINGAPORE
23.3.7 THAILAND
23.3.8 MALAYSIA
23.3.9 INDONESIA
23.3.10 PHILIPPINES
23.3.11 NEW ZEALAND
23.3.12 TAIWAN
23.3.13 VIETNAM
23.3.14 REST OF ASIA-PACIFIC
23.4 SOUTH AMERICA
23.4.1 BRAZIL
23.4.2 ARGENTINA
23.4.3 PERU
23.4.4 REST OF SOUTH AMERICA
23.5 MIDDLE EAST AND AFRICA
23.5.1 SOUTH AFRICA
23.5.2 SAUDI ARABIA
23.5.3 UAE
23.5.4 EGYPT
23.5.5 KUWAIT
23.5.6 ISRAEL
23.5.7 REST OF MIDDLE EAST AND AFRICA
23.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL DIAGNOSTIC REAGENTS MARKET, SWOT AND DBMR ANALYSIS
25 GLOBAL DIAGNOSTIC REAGENTS MARKET, COMPANY PROFILE
25.1 THERMO FISHER SCIENTIFIC
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 QUIDELORTHO CORPORATION
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 ILLUMINA, INC.
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 MERCK KGAA,
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 PERKINELMER INC
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 SHIMADZU CORPORATION
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 HOLOGIC, INC.
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 CYTENA GMBH
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 ABBOTT
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 BIO-RAD LABORATORIES, INC.
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 DANAHER
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 BIOMÉRIEUX
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 QIAGEN
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 GRIFOLS, S.A.
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 FUJIREBIO DIAGNOSTICS AB
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 MERIDIAN BIOSCIENCE
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 EKF DIAGNOSTICS HOLDINGS PLC
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 RANDOX LABORATORIES LTD.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 SEKISUI DIAGNOSTICS
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 BD
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 SIEMENS HEALTHCARE GMBH
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
25.22 F. HOFFMANN-LA ROCHE LTD
25.22.1 COMPANY OVERVIEW
25.22.2 REVENUE ANALYSIS
25.22.3 GEOGRAPHIC PRESENCE
25.22.4 PRODUCT PORTFOLIO
25.22.5 RECENT DEVELOPMENTS
25.23 DIASORIN S.P.A.
25.23.1 COMPANY OVERVIEW
25.23.2 REVENUE ANALYSIS
25.23.3 GEOGRAPHIC PRESENCE
25.23.4 PRODUCT PORTFOLIO
25.23.5 RECENT DEVELOPMENTS
25.24 SYSMEX CORPORATION
25.24.1 COMPANY OVERVIEW
25.24.2 REVENUE ANALYSIS
25.24.3 GEOGRAPHIC PRESENCE
25.24.4 PRODUCT PORTFOLIO
25.24.5 RECENT DEVELOPMENTS
25.25 ORTHO CLINICAL DIAGNOSTICS
25.25.1 COMPANY OVERVIEW
25.25.2 REVENUE ANALYSIS
25.25.3 GEOGRAPHIC PRESENCE
25.25.4 PRODUCT PORTFOLIO
25.25.5 RECENT DEVELOPMENTS
25.26 AGILENT TECHNOLOGIES INC.
25.26.1 COMPANY OVERVIEW
25.26.2 REVENUE ANALYSIS
25.26.3 GEOGRAPHIC PRESENCE
25.26.4 PRODUCT PORTFOLIO
25.26.5 RECENT DEVELOPMENTS
25.27 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD.
25.27.1 COMPANY OVERVIEW
25.27.2 REVENUE ANALYSIS
25.27.3 GEOGRAPHIC PRESENCE
25.27.4 PRODUCT PORTFOLIO
25.27.5 RECENT DEVELOPMENTS
25.28 FUJIFILM
25.28.1 COMPANY OVERVIEW
25.28.2 REVENUE ANALYSIS
25.28.3 GEOGRAPHIC PRESENCE
25.28.4 PRODUCT PORTFOLIO
25.28.5 RECENT DEVELOPMENTS
25.29 BIOSINO BIO-TECHNOLOGY AND SCIENCE INC.
25.29.1 COMPANY OVERVIEW
25.29.2 REVENUE ANALYSIS
25.29.3 GEOGRAPHIC PRESENCE
25.29.4 PRODUCT PORTFOLIO
25.29.5 RECENT DEVELOPMENTS
25.3 DAAN GENE CO., LTD.
25.30.1 COMPANY OVERVIEW
25.30.2 REVENUE ANALYSIS
25.30.3 GEOGRAPHIC PRESENCE
25.30.4 PRODUCT PORTFOLIO
25.30.5 RECENT DEVELOPMENTS
25.31 HIPRO BIOTECHNOLOGY CO.,LTD.
25.31.1 COMPANY OVERVIEW
25.31.2 REVENUE ANALYSIS
25.31.3 GEOGRAPHIC PRESENCE
25.31.4 PRODUCT PORTFOLIO
25.31.5 RECENT DEVELOPMENTS
25.32 TRIVITRON HEALTHCARE
25.32.1 COMPANY OVERVIEW
25.32.2 REVENUE ANALYSIS
25.32.3 GEOGRAPHIC PRESENCE
25.32.4 PRODUCT PORTFOLIO
25.32.5 RECENT DEVELOPMENTS
25.33 DIAGNOSTIC SYSTEMS GMBH
25.33.1 COMPANY OVERVIEW
25.33.2 REVENUE ANALYSIS
25.33.3 GEOGRAPHIC PRESENCE
25.33.4 PRODUCT PORTFOLIO
25.33.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.