Global Pharmacogenetic Testing In Psychiatry Depression Market
Market Size in USD Billion
CAGR :
% 
 USD
1.34 Billion
USD
2.97 Billion
2024
2032
USD
1.34 Billion
USD
2.97 Billion
2024
2032
| 2025 –2032 | |
| USD 1.34 Billion | |
| USD 2.97 Billion | |
|
|
|
|
Pharmacogenetics Testing in Psychiatry/Depression Market Size
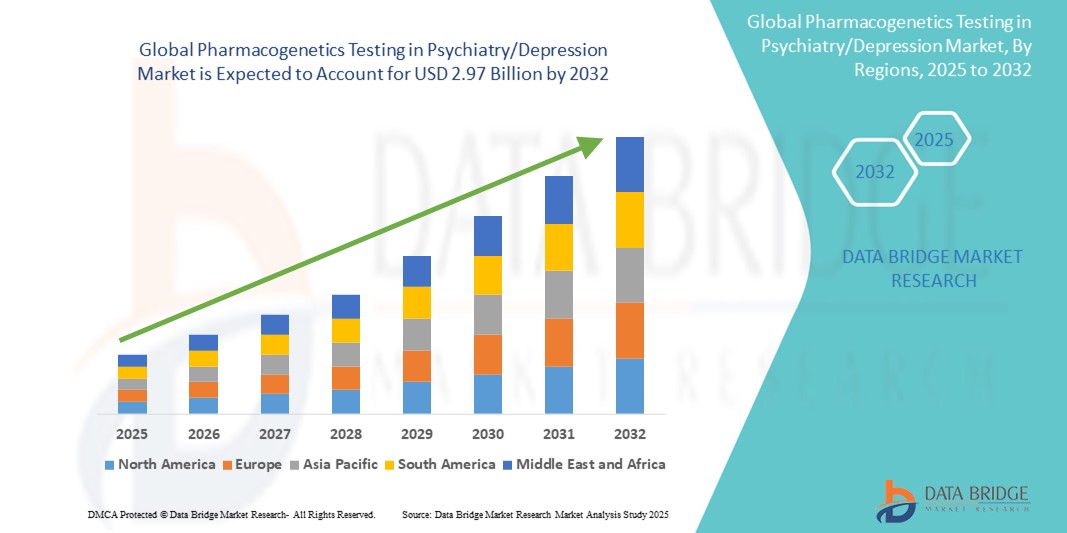
- The global pharmacogenetics testing in psychiatry/depression market size was valued at USD 1.34 billion in 2024 and is expected to reach USD 2.97 billion by 2032, at a CAGR of 10.4% during the forecast period
- The market growth is largely fueled by the increasing adoption of personalized medicine and advancements in genomic technologies, leading to more precise and effective treatment strategies in mental health care. Pharmacogenetics testing in psychiatry and depression enables clinicians to tailor antidepressant and antipsychotic medications based on individual genetic profiles, thereby improving treatment efficacy and reducing adverse drug reactions
- Furthermore, rising awareness among healthcare providers and patients about the benefits of gene-based drug selection is establishing pharmacogenetic testing as a valuable tool in psychiatric care. These converging factors are accelerating the uptake of pharmacogenetics testing in psychiatry/depression solutions, thereby significantly boosting the industry's growth
Pharmacogenetics Testing in Psychiatry/Depression Market Analysis
- Pharmacogenetics testing, which involves analyzing how genetic variations affect a patient’s response to psychiatric medications, is becoming an essential tool in personalized mental healthcare. It enables clinicians to make informed decisions about antidepressant prescriptions, improving treatment outcomes and reducing adverse effects in patients with depression and other psychiatric disorders
- The growing demand for pharmacogenetics testing in psychiatry and depression treatment is primarily driven by the rising prevalence of mental health disorders, increasing awareness among healthcare providers about the benefits of personalized medicine, and expanding support from regulatory bodies for genetic-based therapeutic decisions
- North America dominated the pharmacogenetics testing in psychiatry/depression market with the largest revenue share of 40.6% in 2024, owing to the early adoption of precision medicine, favorable reimbursement policies, and strong presence of leading diagnostic companies. The U.S. in particular has witnessed significant uptake in pharmacogenetic testing due to its integration into psychiatric clinical workflows and increasing use by mental health professionals in both private and public healthcare systems
- Asia-Pacific is projected to be the fastest growing region in the pharmacogenetics testing in psychiatry/depression market during the forecast period, expected to grow at a CAGR of 12.9% from 2025 to 2032, driven by increasing urbanization, rising mental health awareness, government support for genomic research, and improvements in healthcare infrastructure across countries such as China, India, and Japan
- The adult segment held the largest market share of 62.1% in 2024 among all patient types, attributed to the high prevalence of psychiatric conditions in the adult population and the widespread use of pharmacogenetic testing to optimize psychiatric medication regimens and improve treatment outcomes
Report Scope and Pharmacogenetics Testing in Psychiatry/Depression Market Segmentation
|
Attributes |
Pharmacogenetics Testing in Psychiatry/Depression Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pharmacogenetics Testing in Psychiatry/Depression Market Trends
“Rising Demand for Personalized Psychiatry and Depression Treatments”
- A significant and accelerating trend in the global pharmacogenetics testing in psychiatry/depression market is the increasing demand for precision medicine and individualized mental health treatment approaches. This evolution is reshaping how psychiatric disorders are diagnosed and treated, leading to improved therapeutic outcomes
- For instance, pharmacogenetic testing enables clinicians to tailor antidepressant and antipsychotic drug prescriptions based on individual genetic profiles, minimizing adverse drug reactions and enhancing drug efficacy
- The incorporation of genetic biomarkers such as CYP2D6, CYP2C19, and HTR2A/C into diagnostic protocols is allowing healthcare professionals to better predict patient response to psychiatric medications such as SSRIs, SNRIs, and mood stabilizers
- These advancements are particularly beneficial in managing treatment-resistant depression, bipolar disorder, and schizophrenia, where traditional trial-and-error prescribing often delays symptom relief
- Government initiatives across countries such as the U.S., U.K., and Japan are increasingly funding research and implementation of pharmacogenomics in psychiatry, further accelerating market growth
- Furthermore, growing awareness among clinicians and patients about the role of genetics in mental health, along with expanding availability of testing through hospital pharmacies, clinics, and online platforms, is expected to drive widespread adoption in both clinical and outpatient settings
Pharmacogenetics Testing in Psychiatry/Depression Market Dynamics
Driver
“Growing Need Due to Rising Demand for Personalized Psychiatric Care”
- The increasing prevalence of psychiatric disorders such as depression, bipolar disorder, and anxiety, coupled with rising demand for precision medicine, is a significant driver for the heightened demand for pharmacogenetics testing in psychiatry
- For instance, in April 2024, multiple research initiatives globally began focusing on the integration of gene-drug interaction data to improve antidepressant and antipsychotic prescribing practices. Such strategies by key companies and research institutions are expected to drive the Pharmacogenetics Testing in Psychiatry/Depression industry growth during the forecast period
- As clinicians and patients become more aware of variable drug responses caused by genetic differences, pharmacogenetics offers advanced tools such as CYP2D6 and CYP2C19 testing, providing a compelling alternative to traditional trial-and-error medication models
- Furthermore, the growing emphasis on mental health management and personalized treatment pathways is making pharmacogenetic tests an integral component of psychiatric care, supporting integration into clinical decision support systems and EHR platforms
- The benefits of informed drug selection, reduced adverse effects, and improved treatment outcomes are key factors propelling the adoption of pharmacogenetic testing across hospitals, specialty clinics, and diagnostic laboratories. The increasing availability of user-friendly kits and software-driven interpretation platforms further contributes to market expansion
Restraint/Challenge
“Limited Awareness, Reimbursement Barriers, and High Testing Costs”
- Limited awareness among general practitioners and psychiatrists regarding the clinical utility of pharmacogenetics testing remains a significant barrier to widespread adoption, particularly in developing regions
- For instance, despite the growing number of validated gene-drug pairs, inconsistent clinical guidelines and lack of standardized reporting can discourage adoption among healthcare providers
- Reimbursement challenges, particularly in public healthcare systems, further constrain the use of these tests, as many insurance providers do not yet cover comprehensive pharmacogenetic panels
- Moreover, the relatively high initial cost of testing compared to traditional approaches may deter patients and smaller clinics, especially in cost-sensitive markets
- While pricing is expected to become more competitive with technological advancements and increased adoption, the perception of high cost without immediate visible benefits continues to hinder market growth
- Overcoming these challenges through medical education programs, policy reforms supporting reimbursement, and the development of affordable pharmacogenetics testing solutions will be essential for sustained market expansion
Pharmacogenetics Testing in Psychiatry/Depression Market Scope
The market is segmented on the basis of type, product, test type, gene type, patient type, end user, and distribution channel.
• By Type
On the basis of type, the pharmacogenetics testing in psychiatry/depression market is segmented into anxiety, depression, mood disorders, bipolar disorders, eating disorders, and psychotic disorders. The depression segment held the largest market revenue share of 51.4% in 2024, driven by the high global prevalence of major depressive disorder and the strong demand for personalized antidepressant therapy.
The bipolar disorders segment is anticipated to witness the fastest growth rate of 13.8% from 2025 to 2032, supported by increasing research into gene-drug interactions and the rising use of pharmacogenetic tools in mood stabilization treatments.
• By Product
On the basis of product, the pharmacogenetics testing in psychiatry/depression market is segmented into consumables, instruments, and software and services. Consumables dominated the market with the largest revenue share of 45.7% in 2024, due to frequent test kit usage and increasing test volumes.
Software and services are expected to grow at the fastest CAGR of 14.2% from 2025 to 2032, driven by growing demand for interpretation tools, digital test platforms, and analytics in personalized medicine.
• By Test Type
On the basis of test type, the market is segmented into whole genome sequencing and chromosomal array-based tests. Chromosomal array-based tests accounted for the largest market share of 56.3% in 2024, widely adopted due to their affordability and targeted analysis for psychiatric-relevant genes.
Whole genome sequencing is projected to exhibit the fastest growth rate of 15.6% from 2025 to 2032, as costs decline and demand for comprehensive genetic profiling increases.
• By Gene Type
On the basis of gene type, the pharmacogenetics testing in psychiatry/depression market is segmented into CYP2C19, CYP2C9 and VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT, and Others. CYP2D6 held the largest share of 28.4% in 2024, due to its critical role in metabolizing a wide range of psychiatric medications.
HTR2A/C is anticipated to record the fastest CAGR of 12.9% from 2025 to 2032, reflecting growing research linking serotonin receptor genetics to antidepressant response.
• By Patient Type
On the basis of patient type, the pharmacogenetics testing in psychiatry/depression market is segmented into adult, geriatric, and child patient groups. The adult segment dominated with a revenue share of 62.1% in 2024, as adults represent the largest population undergoing psychiatric treatments globally.
The geriatric population is expected to grow at the fastest CAGR of 13.5% from 2025 to 2032, driven by rising mental health awareness and personalized prescribing in elderly care.
• By End User
On the basis of end user, the pharmacogenetics testing in psychiatry/depression market is segmented into hospitals and clinics, diagnostic laboratories, academic and research institutes, and others. Hospitals and Clinics accounted for the largest market share of 46.2% in 2024, owing to integration of pharmacogenetic testing into psychiatric care pathways.
Diagnostic laboratories are projected to expand at the fastest CAGR of 14.7% from 2025 to 2032, due to outsourcing trends and the increasing availability of molecular testing services.
• By Distribution Channel
On the basis of end user, the pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third party distribution, hospital pharmacy, and others. Direct tender dominated with the largest revenue share of 38.5% in 2024, especially from large-scale procurement by healthcare systems and institutions.
Third party distribution is expected to grow at the fastest CAGR of 13.2% from 2025 to 2032, supported by partnerships with diagnostic service providers and independent pharmacy networks.
Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis
- North America dominated the pharmacogenetics testing in psychiatry/depression market with the largest revenue share of 40.6% in 2024, driven by the rising adoption of personalized medicine, favorable reimbursement frameworks, and a well-established healthcare infrastructure
- The region benefits from increasing awareness among clinicians regarding gene-drug interactions and the presence of leading pharmacogenetic testing companies
- Clinicians and healthcare providers in the U.S. and Canada are increasingly leveraging genetic testing to tailor psychiatric medication regimens, leading to improved patient compliance and reduced adverse drug reactions. Continued investments in mental health and genomics research further support the widespread integration of pharmacogenetics into psychiatric practice
U.S. Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The U.S. pharmacogenetics testing in psychiatry/depression market captured the largest revenue share of 81.0% within North America in 2024, driven by the increasing emphasis on precision medicine, high prevalence of mental health disorders, and strong support from organizations such as the FDA and NIH. In addition, the presence of prominent genetic testing companies and mental health-focused startups facilitates broad test accessibility across healthcare settings.
Europe Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The Europe pharmacogenetics testing in psychiatry/depression market is projected to expand at a substantial CAGR throughout the forecast period, fueled by government initiatives promoting personalized psychiatry, rising mental health awareness, and growing integration of genetic testing in treatment guidelines. The region's emphasis on regulatory quality and patient safety fosters trust in pharmacogenetic approaches.
U.K. Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The U.K. pharmacogenetics testing in psychiatry/depression market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the National Health Service (NHS)’s push toward genomics integration in psychiatric care, increased adoption in private clinics, and rising prevalence of depression and anxiety. The nation’s investment in precision medicine initiatives, such as Genomics England, also accelerates market growth.
Germany Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The Germany pharmacogenetics testing in psychiatry/depression market i is expected to expand at a considerable CAGR during the forecast period, supported by a strong research ecosystem, wide availability of genetic testing services, and the country’s leadership in digital health innovation. German healthcare providers are increasingly incorporating gene-based diagnostics into psychiatric medication management, particularly for treatment-resistant depression and bipolar disorder.
Asia-Pacific Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The Asia-Pacific pharmacogenetics testing in psychiatry/depression market is poised to grow at the fastest CAGR of 12.9% from 2025 to 2032, driven by rapid urbanization, expanding healthcare access, rising awareness of mental health conditions, and growing investments in precision medicine. The proliferation of private diagnostic labs and affordable testing options is accelerating adoption across China, India, and Japan.
Japan Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The Japan pharmacogenetics testing in psychiatry/depression market market is witnessing significant growth due to its technologically advanced healthcare infrastructure, an aging population with rising psychiatric care needs, and the integration of pharmacogenetic testing into routine psychiatric evaluations. The adoption of such testing is further supported by government-funded initiatives aimed at promoting genomic research and personalized care.
China Pharmacogenetics Testing in Psychiatry/Depression Market Insight
The China pharmacogenetics testing in psychiatry/depression market accounted for the largest revenue share in Asia-Pacific in 2024, fueled by the country’s growing middle class, high burden of depression, and rapid development of domestic genetic testing capabilities. Government initiatives encouraging genomics adoption in public hospitals and the presence of tech-savvy consumers are boosting the expansion of pharmacogenetics in psychiatric care across major cities.
Pharmacogenetics Testing in Psychiatry/Depression Market Share
The pharmacogenetics testing in psychiatry/depression industry is primarily led by well-established companies, including:
• Thermo Fisher Scientific Inc. (U.S.)
• Illumina Inc. (U.S.)
• Myriad Genetics Inc. (U.S.)
• Sonic Healthcare Limited (Australia)
• QIAGEN (Germany)
• AB-BIOTICS S.A. (Spain)
• BiogeniQ Inc. (Canada)
• Castle Bioscience Inc. (U.S.)
• Coriell Life Sciences (U.S.)
• Dynamic DNA Laboratories (U.S.)
• Eurofins Scientific (Luxembourg)
• Genelex (U.S.)
• Genewiz (U.S.)
• Genomind Inc. (U.S.)
• GenXys (Canada)
• HealthSpek (U.S.)
• HudsonAlpha (U.S.)
• MD Labs (U.S.)
• ONEOME LLC (U.S.)
• PacBio (U.S.)
Latest Developments in Global Pharmacogenetics Testing in Psychiatry/Depression Market
- In October 2024, Agilus Diagnostics announced the launch of its Pharmacogenomics Testing Service. This new offering provides healthcare professionals and patients with valuable insights into how an individual's genetic makeup affects drug response, supporting a more personalized and effective approach to treatment
- In October 2024, Genomind, a leader in personalized medicine and medication management through pharmacogenetics (PGx), announced the launch of its innovative Patient Report. This new report equips patients with clear, accessible insights into how their genetic profile affects their response to various medications
- In February 2023, MyRx—a new UF Health service developed by pharmacists at the University of Florida College of Pharmacy—was launched to offer patients a convenient way to interpret their pharmacogenetic test results and optimize drug therapy. This testing analyzes specific genes to help predict how individuals may respond to certain medications, guiding the selection of the most effective drugs and dosages while minimizing side effects
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
- introduction
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- OVERVIEW of Global pharmacogenetic testing in psychiatry/depression market
- LIMITATIONs
- MARKETS COVERED
- MARKET SEGMENTATION
- MARKETS COVERED
- geographical scope
- years considered for the study
- currency and pricing
- DBMR TRIPOD DATA VALIDATION MODEL
- MULTIVARIATE MODELLING
- type LIFELINE CURVE
- primary interviews with key opinion leaders
- DBMR MARKET POSITION GRID
- market application coverage grid
- vendor share analysis
- secondary sourcEs
- assumptions
- EXECUTIVE SUMMARY
- premium insights
- REGULATIONS: GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- UNITED STATES
- ROLE OF FDA
- ROLE OF CDC AND HCFA
- EUROPEAN UNION
- FRANCE
- AUSTRALIA
- SOUTH KOREA
- market overview
- drivers
- RISING NUMBER OF POPULATION SUFFERING FROM DEPRESSIVE DISORDER
- INITIATIVES TAKEN BY MANUFACTURERS
- GROWING BIOTECHNOLOGY SECTOR ALONG WITH RISING HEALTHCARE EXPENDITURE
- INCREASING INTEREST FOR PERSONALIZED AND PRECISION MEDICATION
- GROWING MEDICAL TOURISM
- RESTRAINTS
- LACK OF STRONG CLINICAL EVIDENCE
- HIGH COST
- LACK OF REIMBURSEMENT
- OPPORTUNITIES
- TECHNOLOGICAL ADVANCEMENTS
- EMERGENCE OF NEW PLAYERS
- Untapped market
- CHALLENGES
- STRINGENT GOVERNMENT REGULATION
- SHORTAGE OF SKILLED PERSONNEL
- COVID-19 IMPACT ON GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
- IMPACT ON PRICE
- IMPACT ON DEMAND
- IMPACT ON SUPPLY
- KEY INITIATIVES BY MARKET PLAYER DURING COVID 19
- CONCLUSION:
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
- overview
- WHOLE GENOME SEQUENCING
- array-based TESTS
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES
- overview
- cyp2c19
- CYP2C9 and VKORC1
- cyp2d6
- HLA-B
- htr2a/c
- HLA-A
- cyp3A4
- slc6a4
- MTHFR
- COMT
- others
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE
- overview
- PRESCRIPTION DRUGS
- Over-the-counter medications
- RECREATIONAL DRUGS
- VITAMINS/NUTRACEUTICals
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE
- overview
- SALIVA
- BLOOD
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY application
- overview
- DRUG DEVELOPMENT
- CLINICAL PRACTICE
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER
- overview
- pharmaceutical and biotechnology companies
- HEALTHCARE PROVIDERS
- research centers and academic institues
- others
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
- overview
- RETAIL PHARMACIES
- HOSPITAL PHARMACIES
- MAIL-ORDER PHARMACIES
- DIRECt-to-customer services
- GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, by Geography
- overview
- north america
- u.s.
- canada
- mexico
- europe
- u.k.
- germany
- france
- italy
- spain
- netherlands
- belgium
- russia
- switzerland
- turkey
- rest of europe
- Asia-Pacific
- JAPAN
- cHINA
- SOUTH KOREA
- INDIA
- AUSTRALIA
- SINGAPORE
- THAILAND
- MALAYSIA
- indonesia
- philipPines
- rest of asia-pacific
- south america
- BRAZIL
- argentina
- rest of south america
- middle east & africa
- south africa
- saudi arabia
- uae
- israel
- egypt
- rest of middle east & africa
- Global Pharmacogenetic Testing in psychiatry/depression Market: COMPANY landscape
- company share analysis: Global
- company share analysis: North America
- company share analysis: Europe
- company share analysis: Asia-Pacific
- swot analysis
- company profile
- MYRIAD GENETICS, INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- Company share analysis
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- THERMO FISHER SCIENTIFIC INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- Company share analysis
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- STADA ARZNEIMITTEL AG
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- Company share analysis
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- sonic healthcare
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- Company share analysis
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- Illumina, Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- Company share analysis
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- AB-Biotics, S.A.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- 6.3 PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- ALTHEADX
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- biogeniq inc.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- Color
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- cnsdose
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CORIELL LIFE SCIENCES
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- Dynamic DNA Laboratories
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- GENELEX
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- genomind, inc.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- genxys
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- HEALTHSPEK
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- HudsonAlpha Health Alliance (A Division of HudsonAlpha)
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- luminex corporation
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- MILLENNIUM HEALTH
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- mydna life australia pty ltd.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- oneome
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- OMECARE
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PerkinElmer Inc.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- R-Biopharm AG
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- questionnaire
- related reports
List of Table
TABLE 1 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, (2021-2028)(USD Million)
TABLE 2 Global Whole Genome Sequencing in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 3 Global Array-Based Tests in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 4 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENES, (2021-2028)(USD Million)
TABLE 5 Global CYP2C19 in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 6 Global CYP2C9 AND VKORC1 in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 7 Global CYP2D6 in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 8 Global HLA-B in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 9 Global HTR2A/C in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 10 Global HLA-A in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 11 Global CYP3A4 in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 12 Global SLC6A4 in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 13 Global MTHFR in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 14 Global COMT in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 15 Global Others in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 16 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DRUG TYPE, (2021-2028)(USD Million)
TABLE 17 Global PRESCRIPTION DRUGS in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 18 Global OVER-THE-COUNTER MEDICATIONS in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 19 Global RECREATIONAL DRUGS in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 20 Global VITAMINS/NUTRACEUTICALS in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 21 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY SAMPLE TYPE, (2021-2028)(USD Million)
TABLE 22 Global Saliva in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 23 Global blood in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 24 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY APPLICATION, (2021-2028)(USD Million)
TABLE 25 Global Drug Development in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 26 Global Clinical Practice in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 27 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, (2021-2028)(USD Million)
TABLE 28 Global Pharmaceutical & Biotechnology Companies in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 29 Global Healthcare Providers in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 30 Global Research Centres And Academic Institutes in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 31 Global Others in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 32 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, (2021-2028)(USD Million)
TABLE 33 Global Retail Pharmacies in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 34 Global Hospital Pharmacies in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 35 Global Mail-Order Pharmacies in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million))
TABLE 36 Global Direct-To-Customer Services in Pharmacogenetic Testing in psychiatry/depression Market, By Region, 2019-2028 (USD Million)
TABLE 37 Global Pharmacogenetic Testing in psychiatry/depression Market , By Region, 2021-2028 (USD Million)
TABLE 38 north america pharmacogenetic testing in psychiatry/depression Market, By COUNTRY, 2021-2028 (USD million)
TABLE 39 north america pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 40 north america pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 41 north america pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 42 north america pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 43 north america pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 44 north america pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 45 north america pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 46 u.s. pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 47 u.s. pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 48 u.s. pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 49 u.s. pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 50 u.s. pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 51 u.s. pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 52 u.s. pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 53 canada pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 54 canada pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 55 canada pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 56 canada pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 57 canada pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 58 canada pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 59 canada pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 60 mexico pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 61 mexico pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 62 mexico pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 63 mexico pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 64 mexico pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 65 mexico pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 66 mexico pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 67 europe pharmacogenetic testing in psychiatry/depression Market, By COUNTRY, 2021-2028 (USD million)
TABLE 68 europe pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 69 europe pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 70 europe pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 71 europe pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 72 europe pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 73 europe pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 74 europe pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 75 u.k. pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 76 u.k. pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 77 u.k. pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 78 u.k. pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 79 u.k. pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 80 u.k. pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 81 u.k. pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 82 germany pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 83 germany pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 84 germany pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 85 germany pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 86 germany pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 87 germany pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 88 germany pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 89 france pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 90 france pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 91 france pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 92 france pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 93 france pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 94 france pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 95 france pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 96 italy pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 97 italy pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 98 italy pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 99 italy pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 100 italy pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 101 italy pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 102 italy pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 103 spain pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 104 spain pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 105 spain pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 106 spain pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 107 spain pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 108 spain pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 109 spain pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 110 netherlands pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 111 netherlands pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 112 netherlands pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 113 netherlands pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 114 netherlands pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 115 netherlands pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 116 netherlands pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 117 belgium pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 118 belgium pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 119 belgium pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 120 belgium pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 121 belgium pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 122 belgium pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 123 belgium pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 124 russia pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 125 russia pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 126 russia pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 127 russia pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 128 russia pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 129 russia pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 130 russia pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 131 switzerland pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 132 switzerland pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 133 switzerland pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 134 switzerland pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 135 switzerland pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 136 switzerland pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 137 switzerland pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 138 turkey pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 139 turkey pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 140 turkey pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 141 turkey pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 142 turkey pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 143 turkey pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 144 turkey pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 145 rest of europe pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 146 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2018-2028 (USD MILLION)
TABLE 147 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 148 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 149 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 150 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 151 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 152 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 153 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 154 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 155 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 156 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 157 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 158 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 159 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 160 JAPAN PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 161 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 162 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 163 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 164 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 165 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 166 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 167 CHINA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 168 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 169 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 170 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 171 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 172 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 173 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 174 SOUTH KOREA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 175 India PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 176 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 177 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 178 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 179 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 180 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 181 INDIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 182 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 183 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 184 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 185 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 186 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 187 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 188 australia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 189 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 190 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 191 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 192 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 193 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 194 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 195 SINGAPORE PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 196 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 197 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 198 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 199 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 200 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 201 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 202 THailand PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 203 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 204 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 205 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 206 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 207 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 208 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 209 MALAYSIA PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 210 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 211 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 212 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 213 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 214 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 215 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 216 indonesia PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 217 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 218 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By GENE, 2021-2028 (USD Million)
TABLE 219 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DRUG Type, 2021-2028 (USD Million)
TABLE 220 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By SAMPLE TYPE, 2021-2028 (USD Million)
TABLE 221 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By APPLICATION, 2021-2028 (USD Million)
TABLE 222 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By END USER, 2021-2028 (USD Million)
TABLE 223 PHILIPPINES PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By DISTRIBUTION CHANNEL, 2021-2028 (USD Million)
TABLE 224 Rest of Asia-Pacific PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET, By Type, 2021-2028 (USD Million)
TABLE 225 south america pharmacogenetic testing in psychiatry/depression Market, By COUNTRY, 2021-2028 (USD million)
TABLE 226 south america pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 227 south america pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 228 south america pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 229 south america pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 230 south america pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 231 south america pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 232 south america pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 233 brazil pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 234 brazil pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 235 brazil pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 236 brazil pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 237 brazil pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 238 brazil pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 239 brazil pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 240 argentina pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 241 argentina pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 242 argentina harmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 243 argentina pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 244 argentina pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 245 argentina pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 246 argentina pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 247 rest of south america pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 248 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By COUNTRY, 2021-2028 (USD million)
TABLE 249 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 250 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 251 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 252 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 253 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 254 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 255 middle east & africa pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 256 south africa pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 257 south africa pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 258 south africa pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 259 south africa pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 260 south africa pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 261 south africa pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 262 south africa pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 263 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 264 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 265 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 266 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 267 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 268 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 269 saudi arabia pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 270 uae pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 271 uae pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 272 uae pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 273 uae pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 274 uae pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 275 uae pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 276 uae pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 277 israel pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 278 israel pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 279 israel pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 280 israel pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 281 israel pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 282 israel pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 283 israel pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 284 egypt pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
TABLE 285 egypt pharmacogenetic testing in psychiatry/depression Market, By genes, 2021-2028 (USD million)
TABLE 286 egypt pharmacogenetic testing in psychiatry/depression Market, By drug type, 2021-2028 (USD million)
TABLE 287 egypt pharmacogenetic testing in psychiatry/depression Market, By sample, 2021-2028 (USD million)
TABLE 288 EGYPT pharmacogenetic testing in psychiatry/depression Market, By application, 2021-2028 (USD million)
TABLE 289 egypt pharmacogenetic testing in psychiatry/depression Market, By end user, 2021-2028 (USD million)
TABLE 290 egypt pharmacogenetic testing in psychiatry/depression Market, By distribution channel, 2021-2028 (USD million)
TABLE 291 rest of middle east & africa pharmacogenetic testing in psychiatry/depression Market, By type, 2021-2028 (USD million)
List of Figure
FIGURE 1 Global PHARMACOGENTIC TESTIG IN PSYCHIATRY/DEPRESSION market: segmentation
FIGURE 2 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: data triangulation
FIGURE 3 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: DROC ANALYSIS
FIGURE 4 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: global vs REGIONAL MARKET ANALYSIS
FIGURE 5 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: COMPANY RESEARCH ANALYSIS
FIGURE 6 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: INTERVIEW DEMOGRAPHICS
FIGURE 7 Global PHARMACOGENETIC TESTING IN PSYCHIATRIC/DEPRESSION market: DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL pharmacogenetic testing in psychiatry/depression MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 Global pharmacogenetic testing in psychiatry/depression market: vendor share analysis
FIGURE 10 Global pharmacogenetic testing in psychiatry/depression market: SEGMENTATION
FIGURE 11 initiatives taken by manufacturers is expected to drive THE Global pharmacogenetic testing in psychiatry/depression market IN THE FORECAST PERIOD of 2021 to 2028
FIGURE 12 whole genome sequencing segment is expected to account for the largest share of the Global pharmacogenetic testing in psychiatry/depression market in 2021 & 2028
FIGURE 13 NORTH AMERICA is expected to DOMINATE the gLOBAL pharmacogenetic testing in psychiatry/depression market and ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 14 asia-pacific is the fastest growing market for pharmacogenetic testing in psychiatry/depression manufacturers in the forecast period of 2021 to 2028
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET
FIGURE 16 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021-2028
FIGURE 17 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021-2028 (USD MILLION)
FIGURE 18 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 19 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2021-2028
FIGURE 21 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, 2021-2028 (USD MILLION)
FIGURE 22 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, CAGR (2021-2028)
FIGURE 23 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENES, LIFELINE CURVE
FIGURE 24 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2021-2028
FIGURE 25 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, 2021-2028 (USD MILLION)
FIGURE 26 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 27 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 28 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2021-2028
FIGURE 29 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, 2021-2028 (USD MILLION)
FIGURE 30 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 31 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY SAMPLE TYPE, LIFELINE CURVE
FIGURE 32 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2021-2028
FIGURE 33 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, 2021-2028 (USD MILLION)
FIGURE 34 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 35 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 36 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2021-2028
FIGURE 37 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, 2021-2028 (USD MILLION)
FIGURE 38 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, CAGR (2021-2028)
FIGURE 39 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021-2028
FIGURE 41 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021-2028 (USD MILLION)
FIGURE 42 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 43 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 45 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY Region (2020)
FIGURE 46 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY Region (2021 & 2028)
FIGURE 47 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY Region (2020 & 2028)
FIGURE 48 GLOBAL PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 49 north america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 50 north america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 51 north america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 52 north america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 53 north america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 54 europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 55 europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 56 europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 57 europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 58 europe PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 59 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 60 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 61 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 62 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 63 ASIA-PACIFIC PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 64 south america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 65 south america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 66 south america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 67 south america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 68 south america PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 69 middle east & africa PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2020)
FIGURE 70 middle east & africa PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020)
FIGURE 71 middle east & africa PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2028)
FIGURE 72 middle east & africa PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2020 & 2028)
FIGURE 73 middle east & africa PHARMACOGENETIC TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY type (2021-2028)
FIGURE 74 Global Pharmacogenetic testing in psychiatry/depression Market: company share 2020 (%)
FIGURE 75 North America pharmacogenetic testing in psychiatry/depression Market: company share 2020 (%)
FIGURE 76 Europe pharmacogenetic testing in psychiatry/depression Market: company share 2020 (%)
FIGURE 77 Asia-Pacific pharmacogenetic testing in psychiatry/DEPRESSION MARKET: company share 2020 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.