Endoscope reprocessing is indispensable in healthcare settings, serving as a frontline defense against cross-contamination. With multiple procedures conducted daily, the risk of infection transmission between patients is a constant concern. Robust reprocessing protocols involving meticulous cleaning and disinfection of endoscopes are vital for breaking the chain of potential infections. The diligence in maintaining a sterile endoscope environment protects individual patients and contributes significantly to the broader goal of enhancing healthcare safety and quality
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-endoscope-reprocessing-market
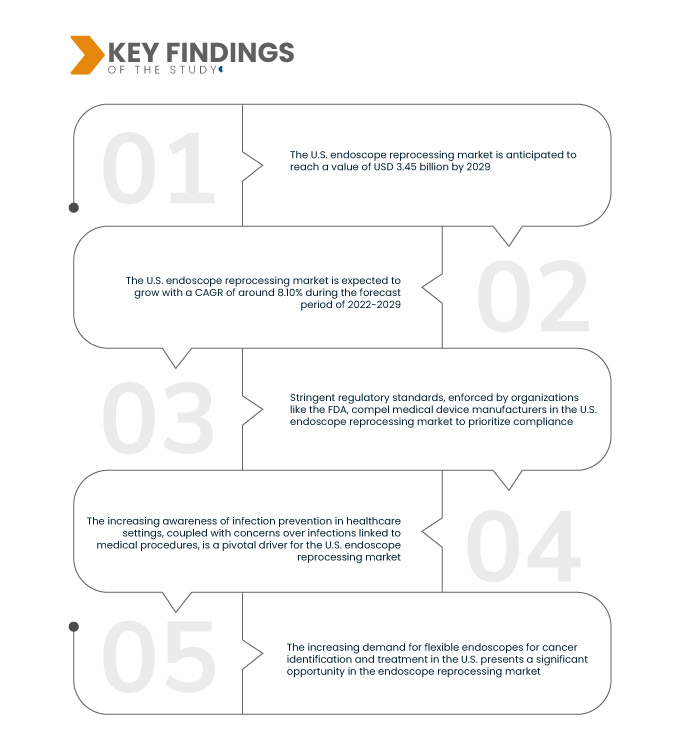
Data Bridge Market Research analyses the U.S. Endoscope Reprocessing Market, which was USD 1.85 billion in 2021, is expected to reach up to USD 3.45 billion by 2029, and is expected to undergo a CAGR of 8.10% during the forecast period 2022 to 2029. The escalating demand for endoscopic procedures in the U.S., driven by their minimally invasive nature, has spurred healthcare providers to adopt these techniques for diagnostics and therapy.
Key Findings of the Study
Increasing healthcare expenditure in the U.S. is expected to drive the market's growth rate
The escalating healthcare expenditure in the U.S. is a pivotal driver for the endoscope reprocessing market. The increased financial allocation to the healthcare sector empowers facilities to invest in cutting-edge medical equipment, particularly advanced endoscope reprocessing systems. This surge in funding underscores a commitment to infection prevention and patient safety. With a willingness to allocate substantial resources, healthcare facilities are propelled to adopt state-of-the-art reprocessing technologies, fostering market expansion.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2022 to 2029
|
Base Year
|
2021
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
Segments Covered
|
Products (Equipments and Consumables), Modality (Bench Top Endoscopic Reprocessors and Stand- Alone Endoscopic Reprocessors), Process Type (Automated Cleaning Disinfectants and Manual Cleaning Solutions), End-User (Hospitals, Ambulatory Surgical Centres (ASCS), Clinics, Diagnostic Centres and Others)
|
Market Players Covered
|
Cantel Medical(美國)、ASP(美國)、OlympU.S. Corporation(日本)、Ecolab(美國)、STERIS(愛爾蘭)、Getinge AB(瑞典)、Wassenburg Medical(荷蘭)、CONMED Corporation(美國)、Belimed AG(瑞士)、Endo-Technik W. Griesat(德國)、CUS.S.B.S.S. Group of Companies Inc.(加拿大)、Metrex Research, LLC。 (加拿大)、Richard Wolf GmbH(德國)、MEDALKAN(希臘)、Micro-Scientific, LLC(美國)、Borer Chemie AG(瑞士)、Tuttnauer(荷蘭)、ATMS(加拿大)、Summit Imaging, Inc.(美國)、Medonica Co. LTD(韓國)、SHIN]
|
報告涵蓋的數據點
|
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
|
細分分析:
美國內視鏡再處理市場根據產品、方式、工藝類型和最終用戶進行細分。
- 根據產品,美國內視鏡再處理市場分為設備和耗材
- 根據模式,美國內視鏡再處理市場分為桌上型內視鏡再處理器及獨立內視鏡再處理器
- 根據製程類型,美國內視鏡再處理市場分為自動清潔消毒劑和手動清潔溶液
- 根據最終用戶,美國內視鏡再處理市場分為醫院、門診手術中心 (ASCS)、診所、診斷中心和其他
主要參與者
Data Bridge Market Research 認為以下公司是美國內視鏡再處理市場的主要參與者,它們是 Cantel Medical(美國)、ASP(美國)、OlympU.S。 Corporation(日本)、Ecolab(美國)、STERIS(愛爾蘭)、Getinge AB(瑞典)、Wassenburg Medical(荷蘭)、CONMED Corporation(美國)、Belimed AG(瑞士)、Endo-Technik W. Griesat(德國)
市場發展
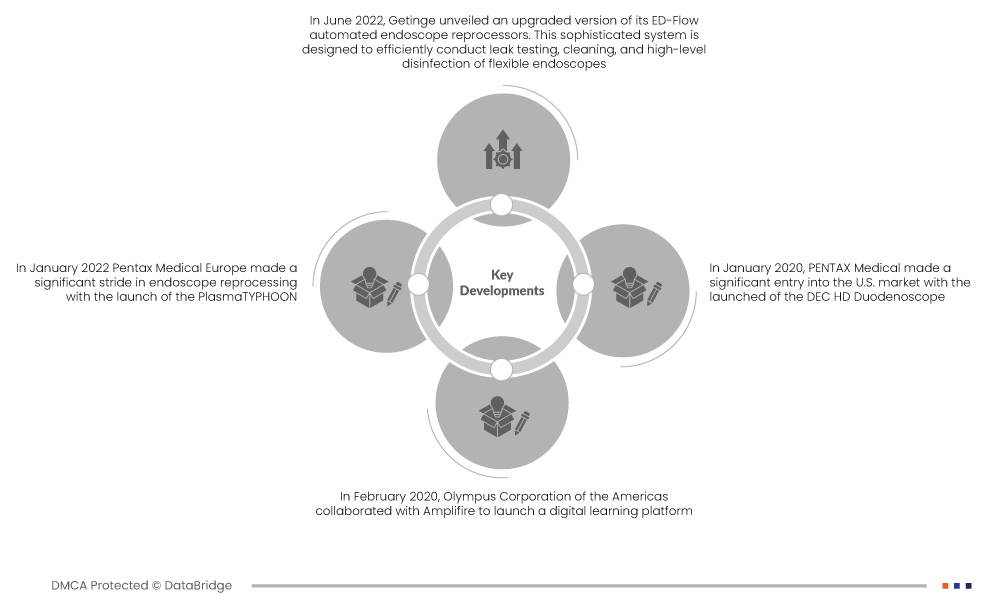
- 2022 年 6 月,Getinge 推出了 ED-Flow 自動內視鏡再處理器的升級版。此先進的系統旨在高效地進行柔性內視鏡的洩漏測試、清潔和高水準消毒。透過專注於提供有效可靠的結果,這項創新有助於維持高標準的醫療設備衛生
- 2022 年 1 月,Pentax Medical Europe 推出 PlasmaTYPHOON,在內視鏡再處理方面取得了重大進展。此尖端解決方案旨在徹底改變內視鏡乾燥和儲存方式,具有超快的功能。該設備不僅提高了生產力和可追溯性,而且在推進內視鏡再處理以增強病患安全方面發揮了關鍵作用
- 2020年2月,奧林巴斯美洲公司與Amplifire合作推出數位學習平台。該平台專為從事內視鏡再處理的技術人員量身定制,提供一種全新、高效的學習方式。該計劃的核心是奧林巴斯 TJF-Q180V 十二指腸鏡的再處理,這是一種用於內視鏡逆行胰膽管造影術 (ERCP) 的複雜醫用內視鏡
- 2020 年 1 月,賓得醫療推出 DEC HD 十二指腸鏡,正式進軍美國市場。這種先進的醫療設備具有高清功能,並包含各種一次性組件以供設備再處理。這些組件中值得注意的是無菌遠端蓋和升降桿,強調了對改善內視鏡再處理衛生實踐的承諾
有關美國內視鏡再處理市場 報告的更多詳細信息,請點擊此處 - https://www.databridgemarketresearch.com/reports/us-endoscope-reprocessing-market