Die steigende Prävalenz von Autoimmunerkrankungen ist ein entscheidender Treiber für den globalen Markt für Anti-Nuklear-Antikörper (ANA)-Tests. Autoimmunerkrankungen wie Lupus, rheumatoide Arthritis und Multiple Sklerose treten aufgrund einer Kombination aus genetischen , umweltbedingten und Lebensstilfaktoren immer häufiger auf. Dieser Anstieg der Prävalenz veranlasst Gesundheitsdienstleister, einer frühen und genauen Diagnose Priorität einzuräumen, um die Behandlungsergebnisse der Patienten zu verbessern. Mit dem wachsenden Bewusstsein für diese Krankheiten erkennen sowohl Patienten als auch medizinisches Fachpersonal eher Symptome und lassen sich diagnostisch testen. Der ANA-Test dient als grundlegendes Instrument zur Identifizierung dieser Erkrankungen und ermöglicht ein rechtzeitiges Eingreifen und eine rechtzeitige Behandlung. Die Bedeutung einer frühzeitigen Erkennung wird durch Studien unterstrichen, die eine schnelle Diagnose mit einem besseren Krankheitsmanagement und einer verbesserten Lebensqualität der Patienten in Verbindung bringen. Darüber hinaus hat die steigende Prävalenz von Autoimmunerkrankungen zu einem stärkeren Fokus auf Forschung und Entwicklung in diesem Bereich geführt, wobei Pharmaunternehmen und Forschungseinrichtungen massiv in die Entdeckung neuer Diagnosemethoden und Behandlungen investieren.
Vollständigen Bericht abrufen: https://www.databridgemarketresearch.com/reports/global-antinuclear-antibody-test-market
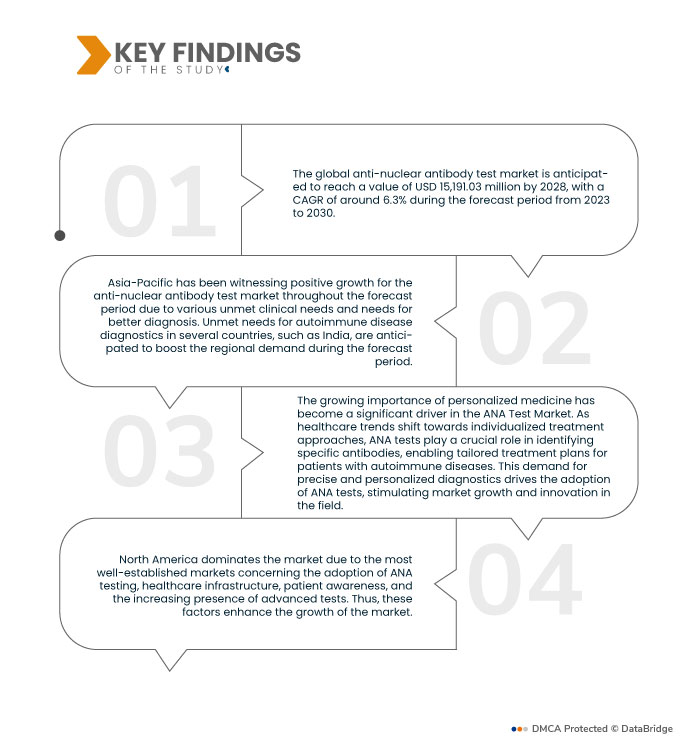
Der globale Markt für Anti-Atom-Antikörpertests hatte im Jahr 2023 einen Wert von 2,65 Milliarden US-Dollar und soll bis 2031 einen Wert von 7,18 Milliarden US-Dollar erreichen, was einem CAGR von 13,3 % im Prognosezeitraum von 2024 bis 2031 entspricht.
Wichtigste Ergebnisse der Studie
Wachsende Forschungsanstrengungen im Bereich Autoimmunerkrankungen treiben das Marktwachstum voran
Wachsende Forschungsanstrengungen im Bereich Autoimmunerkrankungen treiben den globalen Markt für Anti-Atomkraft-Antikörpertests maßgeblich voran. Es fließen mehr Gelder und Ressourcen in die Erforschung der komplexen Mechanismen, die diesen Erkrankungen zugrunde liegen und genetische, umweltbedingte und immunologische Faktoren einschließen. Diese verstärkte Fokussierung führt zur Identifizierung neuer Biomarker und potenzieller therapeutischer Ziele, die die Entwicklung präziserer Diagnoseinstrumente vorantreiben. Indem Forscher neue Erkenntnisse gewinnen, tragen sie zu verbesserten Methoden für ANA-Tests bei und ermöglichen so eine frühere Diagnose und ein besseres Patientenmanagement. Da das Bewusstsein für diese Erkrankungen stetig wächst, bereichert die Forschung nicht nur das wissenschaftliche Wissen, sondern unterstützt auch die Weiterentwicklung der Diagnosemöglichkeiten in der Autoimmunmedizin . Dieses anhaltende Engagement für das Verständnis von Autoimmunerkrankungen korreliert direkt mit einer erhöhten Nachfrage nach ANA-Tests in der klinischen Praxis.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2024 bis 2031
|
Basisjahr
|
2023
|
Historische Jahre
|
2022 (Anpassbar auf 2016–2021)
|
Quantitative Einheiten
|
Umsatz in Milliarden USD
|
Abgedeckte Segmente
|
Antikörpertyp (Extrahierbare nukleäre Antigene (ENA), Anti-DSDNA und Histone, Anti-DFS70-Antikörper, Anti-PM-SCL, Anti-Centromere-Antikörper, Anti-SP100 und andere), Produkt (Instrumente, Verbrauchsmaterialien und Reagenzien sowie Dienstleistungen), Technik (ELISA, Indirekte Immunfluoreszenz (IIF), Blotting-Test, Antigen-Microarray, Gel-basierte Techniken, Multiplex-Assay, Durchflusszytometrie, Passive Hämagglutination (PHA) und andere), Anwendung (Autoimmunerkrankungen und Infektionskrankheiten), nach Endbenutzer (Krankenhäuser, Labore, Diagnosezentren, Forschungsinstitute und andere), Vertriebskanal (Direktausschreibung, Einzelhandel und Drittanbieter und andere)
|
Abgedeckte Länder
|
USA, Kanada, Mexiko, Deutschland, Frankreich, Großbritannien, Italien, Spanien, Russland, Türkei, Belgien, Niederlande, Schweiz, Restliches Europa, China, Indien, Japan, Südkorea, Australien, Singapur, Malaysia, Thailand, Indonesien, Philippinen, Restlicher Asien-Pazifik-Raum, Brasilien, Argentinien, Kolumbien, Venezuela, Restliches Südamerika, Südafrika, Saudi-Arabien, Vereinigte Arabische Emirate, Ägypten, Kuwait, Israel, Bahrain, Oman und Restlicher Naher Osten und Afrika
|
Abgedeckte Marktteilnehmer
|
Thermo Fisher Scientific Inc. (USA), Bio-Rad Laboratories, Inc. (USA), Abbott Laboratories (USA), Euroimmun Medizinische Labordiagnostika AG (Deutschland), Revvity Inc. (USA), Trinity Biotech (Irland), LIFESPAN BIOSCIENCES, INC (USA), ORIGENE TECHNOLOGIES, INC. (USA), Abnova Corporation (Taiwan), CUSABIO TECHNOLOGY LLC (USA), Biorbyt Ltd. (England)
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse:
Der globale Markt für Anti-Atom-Antikörpertests ist nach Antikörpertyp, Produkt, Technik, Anwendung, Endbenutzer und Vertriebskanal segmentiert.
- Auf der Grundlage des Antikörpertyps ist der globale Markt für Anti-Atom-Antikörpertests in Extrahierbare Kernantigene (ENA), Anti-DSDNA & Histone, Anti-DFS70-Antikörper, Anti-PM-SCL, Anti-Centromere-Antikörper, Anti-SP100 und andere unterteilt.
Im Jahr 2024 wird das Segment Extrahierbare Nukleare Antigene (ENA) voraussichtlich den globalen Markt für Anti-Atom-Antikörpertests dominieren
Im Jahr 2024 wird das Segment Extrahierbare Kernantigene (ENA) aufgrund der hohen Prävalenz von Autoimmunerkrankungen voraussichtlich mit 33,07 % den Markt dominieren .
- Der globale Markt für Anti-Atom-Antikörpertests ist auf Produktbasis in Instrumente, Verbrauchsmaterialien und Reagenzien sowie Dienstleistungen unterteilt.
Im Jahr 2024 wird das Instrumentensegment voraussichtlich den globalen Markt für Anti-Atom-Antikörpertests dominieren
Im Jahr 2024 wird das Instrumentensegment voraussichtlich mit einem Marktanteil von 64,37 % den Markt dominieren, da die zunehmende Verbreitung von Autoimmunerkrankungen wie systemischem Lupus erythematodes (SLE), rheumatoider Arthritis (RA) und Sjögren-Syndrom die Nachfrage nach ANA-Tests ankurbelt.
- Der globale Markt für Anti-Atom-Antikörpertests ist nach Technologie segmentiert in ELISA, Indirekte Immunfluoreszenz (IIF), Blotting-Test, Antigen-Microarray, Gel-basierte Verfahren, Multiplex-Assay, Durchflusszytometrie, Passive Hämagglutination (PHA) und weitere. Im Jahr 2024 wird das ELISA-Segment voraussichtlich mit einem Marktanteil von 36,91 % den Markt dominieren.
- Der globale Markt für Anti-Atom-Antikörpertests ist nach Anwendung in Autoimmunerkrankungen und Infektionskrankheiten unterteilt. Im Jahr 2024 wird das Segment der Autoimmunerkrankungen voraussichtlich mit einem Marktanteil von 90,94 % den Markt dominieren.
- Der globale Markt für Anti-Atom-Antikörpertests ist nach Endverbraucher segmentiert in Krankenhäuser, Labore, Diagnosezentren, Forschungsinstitute und weitere. Im Jahr 2024 wird das Krankenhaussegment voraussichtlich mit einem Marktanteil von 45,99 % den Markt dominieren.
- Der globale Markt für Anti-Atom-Antikörpertests ist nach Vertriebskanälen in Direktausschreibungen, Einzelhandelsverkäufe, Drittanbieter und andere unterteilt. Im Jahr 2024 wird das Direktausschreibungssegment voraussichtlich mit 51,20 % Marktanteil den Markt dominieren.
Hauptakteure
Data Bridge Market Research analysiert Thermo Fisher Scientific Inc. (USA), Bio-Rad Laboratories, Inc. (USA), Abbott Laboratories (USA) und Euroimmun Medizinische Labordiagnostika AG (Deutschland) als wichtige Marktteilnehmer in diesem Markt.
Marktentwicklungen
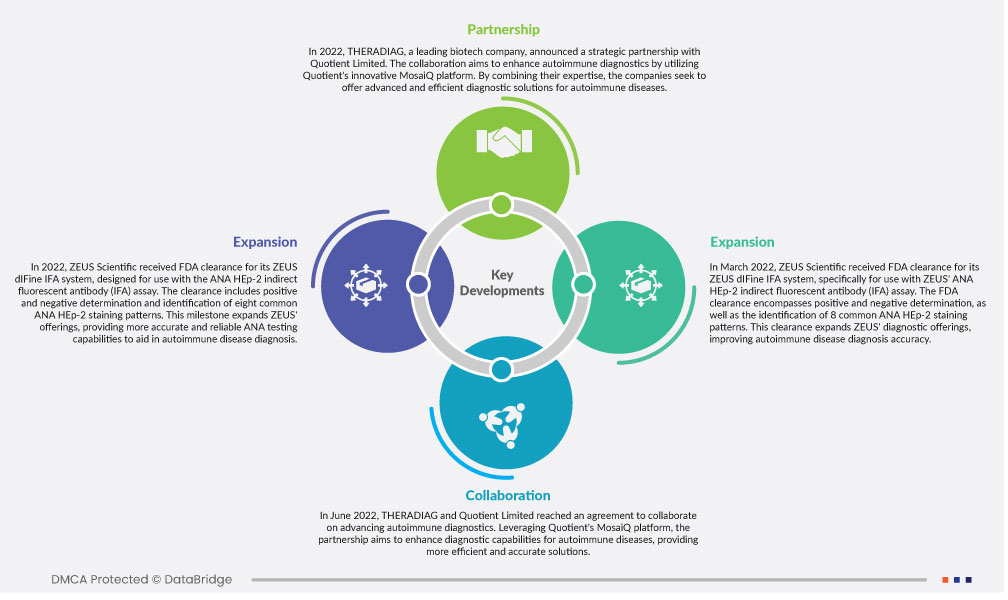
- Im November 2022 hat Bio-Rad sein Angebot an Qualitätskontrollen speziell für die klinischen Diagnostikplattformen von Abbott erweitert und so die Leistung und Zuverlässigkeit der Labore verbessert. Diese Initiative verbessert nicht nur die diagnostische Genauigkeit und die Patientenversorgung, sondern stärkt auch die Wettbewerbsposition von Bio-Rad im Gesundheitsmarkt. Mit innovativen Qualitätskontrolllösungen will Bio-Rad den wachsenden Anforderungen der Labore gerecht werden, das Umsatzwachstum steigern und sein Engagement für die Weiterentwicklung diagnostischer Exzellenz unterstreichen.
- Im August 2022 übernahm Bio-Rad Curiosity Diagnostics, um seine Kapazitäten im Diagnostikmarkt zu erweitern. Die Übernahme stärkt das Portfolio von Bio-Rad durch die Integration innovativer molekularer Testlösungen und ermöglicht es dem Unternehmen, sein Angebot zu erweitern und die Patientenversorgung zu verbessern. Durch die Nutzung der Technologie von Curiosity Diagnostics will Bio-Rad sein Wachstum vorantreiben und seine Position im wettbewerbsintensiven Diagnostikmarkt ausbauen.
Regionale Analyse
Geografisch betrachtet sind dies die folgenden Länder, die im globalen Marktbericht für Anti-Atom-Antikörpertests abgedeckt sind: die USA, Kanada, Mexiko, Deutschland, Frankreich, Großbritannien, Italien, Spanien, Russland, die Türkei, Belgien, die Niederlande, die Schweiz, das übrige Europa, China, Indien, Japan, Südkorea, Australien, Singapur, Malaysia, Thailand, Indonesien, die Philippinen, der übrige asiatisch-pazifische Raum, Brasilien, Argentinien, Kolumbien, Venezuela, der übrige Südamerika, Südafrika, Saudi-Arabien, die Vereinigten Arabischen Emirate, Ägypten, Kuwait, Israel, Bahrain, Oman sowie der übrige Nahe Osten und Afrika.
Laut Marktforschungsanalyse von Data Bridge:
Es wird erwartet, dass die Region Nordamerika den globalen Markt für Anti-Atom-Antikörpertests dominieren wird
Aufgrund der erheblichen Belastung durch Autoimmunerkrankungen wie systemischen Lupus erythematodes (SLE), rheumatoide Arthritis (RA), Sjögren-Syndrom und Typ-1-Diabetes wird Nordamerika voraussichtlich den Markt für Anti-Atom-Antikörpertests dominieren.
Asien-Pazifik ist die am schnellsten wachsende Region im globalen Markt für Anti-Atom-Antikörpertests
Der asiatisch-pazifische Raum ist aufgrund der Expansion von Diagnosezentren und Laboren die am schnellsten wachsende Region auf dem Markt.
Für detailliertere Informationen zum globalen Marktbericht für Anti-Atom-Antikörpertests klicken Sie hier – https://www.databridgemarketresearch.com/reports/global-antinuclear-antibody-test-market