Die Diagnostik des Multiplen Myeloms umfasst wichtige Komponenten wie Geräte und Verbrauchsmaterialien, einschließlich Reagenzien. Dieser Markt, der in die Unterdrückung und Behandlung des Multiplen Myeloms unterteilt ist, verfolgt einen strategischen Ansatz, um die Komplexität der Erkrankung zu bewältigen. Innovationen bei Diagnosegeräten und die Verfügbarkeit wichtiger Verbrauchsmaterialien tragen zu einer effektiven Behandlung bei. Die Fokussierung auf vielfältige Produkttypen unterstreicht das Engagement des Marktes für ganzheitliche Lösungen für eine präzise Diagnose und therapeutische Interventionen beim Multiplen Myelom.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/north-america-multiple-myeloma-diagnostic-market
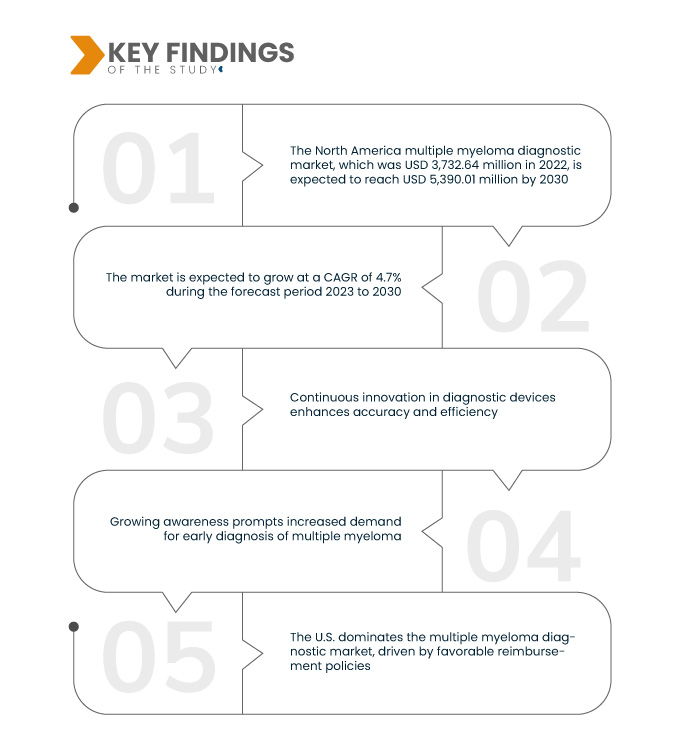
Data Bridge Market Research analysiert, dass der nordamerikanische Markt für Multiple-Myelom-Diagnostik , der im Jahr 2022 3.732,64 Millionen US-Dollar betrug, bis 2030 voraussichtlich 5.390,01 Millionen US-Dollar erreichen wird, was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 4,7 % im Prognosezeitraum 2023 bis 2030 entspricht. Die zunehmende Verbreitung des Multiplen Myeloms ist ein wichtiger Treiber im Diagnostikmarkt. Der Anstieg der Erkrankungshäufigkeit erhöht die Nachfrage nach diagnostischen Interventionen und unterstreicht die zentrale Rolle des Marktes bei der Bewältigung der wachsenden Herausforderungen im Zusammenhang mit dem Multiplen Myelom.
Wichtigste Ergebnisse der Studie
Es wird erwartet, dass die ältere Bevölkerungsgruppe die Wachstumsrate des Marktes vorantreibt
Demografische Trends, insbesondere die alternde Bevölkerung, treiben den Markt für Multiple-Myelom-Diagnostik deutlich voran. Mit der wachsenden Zahl älterer Menschen steigt auch die Anfälligkeit für Multiples Myelom, was effektivere Diagnoselösungen erforderlich macht. Die zentrale Rolle des Marktes liegt darin, die gestiegene Nachfrage nach präziser und zeitnaher Diagnostik zu decken, den Gesundheitsbedürfnissen einer alternden Bevölkerung gerecht zu werden und zu verbesserten Behandlungsergebnissen und einer besseren Behandlung des Multiplen Myeloms in diesem Bevölkerungssegment beizutragen.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2023 bis 2030
|
Basisjahr
|
2022
|
Historische Jahre
|
2021 (Anpassbar auf 2015–2020)
|
Quantitative Einheiten
|
Umsatz in Millionen USD, Mengen in Einheiten, Preise in USD
|
Abgedeckte Segmente
|
Produkttypen (Unterdrückung des multiplen Myeloms und Behandlung des multiplen Myeloms), Typ (Geräte, Verbrauchsmaterialien und Reagenzien), Krankheit (schwelendes (indolentes) multiples Myelom, aktives (symptomatisches) multiples Myelom, solitäres Plasmozytom des Knochens, extramedulläres Plasmozytom, Leichtkettenmyelom, nicht-sekretorisches Myelom, seltene Arten des multiplen Myeloms), Testtyp (Bluttests, Urintests, Knochenmarktests und Genomsequenzierung bei multiplem Myelom), Endbenutzer (Krankenhäuser, Fachkliniken, häusliche Pflege, akademische und Forschungsinstitute, sonstige), Vertriebskanal (Direktausschreibung, Einzelhandel)
|
Abgedeckte Länder
|
USA, Kanada und Mexiko in Nordamerika.
|
Abgedeckte Marktteilnehmer
|
NeoGenomics Laboratories (USA), Helena Laboratories Corporation (USA), Bracco Diagnostic Inc. (Italien), Cytognos SL (Spanien), ZYTOVISION GmbH (Deutschland), Adaptive Biotechnologies (USA), BioVendor Group (USA), TriVitron Healthcare (USA), Eurofins Scientific (Luxemburg), Quest Diagnostic Incorporated (USA), BD (USA), Invivoscribe, Inc. (USA), SkylineDx (Niederlande), Siemens Healthcare GmbH (Deutschland), Thermo Fisher Scientific Inc. (USA) und Laboratory Corporation of America Holdings (USA), GenPath (USA)
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure umfassen die von Data Bridge Market Research kuratierten Marktberichte auch ausführliche Expertenanalysen, Patientenepidemiologie, Pipeline-Analysen, Preisanalysen und regulatorische Rahmenbedingungen.
|
Segmentanalyse:
Der nordamerikanische Markt für die Diagnostik des multiplen Myeloms ist nach Produkttypen, Krankheit, Testtyp, Typ, Endbenutzer und Vertriebskanal segmentiert.
- Auf der Grundlage der Produkttypen ist der nordamerikanische Markt für die Diagnostik des multiplen Myeloms in die Bereiche Unterdrückung des multiplen Myeloms und Behandlung des multiplen Myeloms unterteilt.
- Auf der Grundlage der Krankheit ist der nordamerikanische Markt für die Diagnostik des multiplen Myeloms in schwelendes (indolentes) multiples Myelom, aktives (symptomatisches) multiples Myelom, solitäres Plasmozytom des Knochens, extramedulläres Plasmozytom, Leichtkettenmyelom, nicht-sekretorisches Myelom und seltene Formen des multiplen Myeloms segmentiert.
- Auf der Grundlage des Testtyps ist der nordamerikanische Markt für die Diagnostik des multiplen Myeloms in Bluttests, Urintests, Knochenmarktests und Genomsequenzierung bei multiplem Myelom segmentiert
- Der nordamerikanische Markt für die Diagnostik des multiplen Myeloms ist nach Typ in Geräte und Verbrauchsmaterialien sowie Reagenzien segmentiert.
- Auf der Grundlage des Endverbrauchers ist der nordamerikanische Markt für die Diagnose des multiplen Myeloms in Krankenhäuser, Fachkliniken, häusliche Pflege, akademische und Forschungsinstitute und andere unterteilt
- Auf der Grundlage des Vertriebskanals ist der nordamerikanische Markt für Multiple-Myelom-Diagnostika in Direktausschreibungen und Einzelhandelsverkäufe unterteilt
Hauptakteure
Data Bridge Market Research erkennt die folgenden Unternehmen als Akteure auf dem nordamerikanischen Markt für Multiples Myelomdiagnostik an: NeoGenomics Laboratories (USA), Helena Laboratories Corporation (USA), Bracco Diagnostic Inc. (Italien), Cytognos SL (Spanien), ZYTOVISION GmbH (Deutschland) und Adaptive Biotechnologies (USA).
Marktentwicklungen
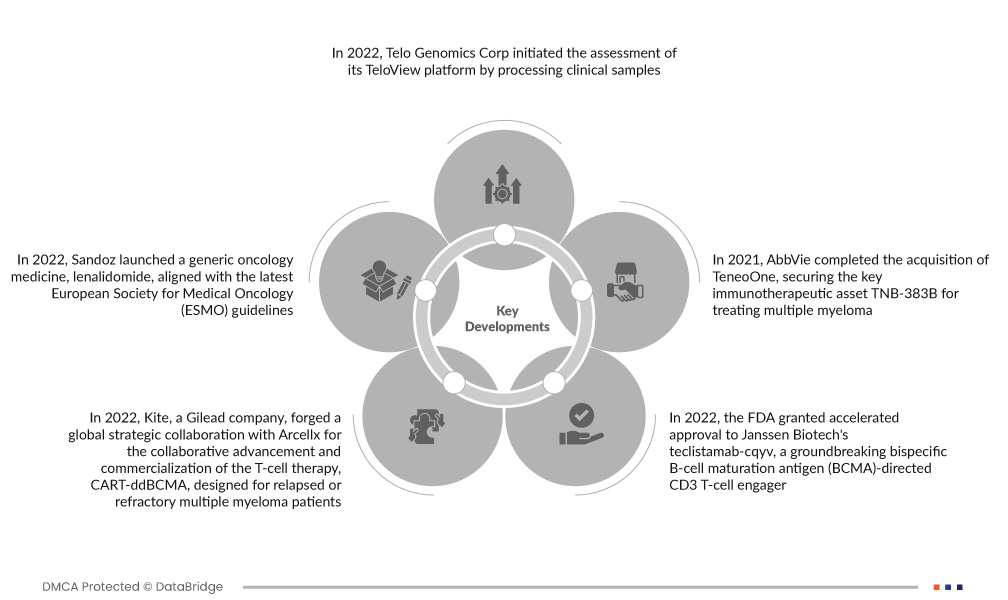
- Im Jahr 2022 begann Telo Genomics Corp. mit der Evaluierung seiner TeloView-Plattform anhand klinischer Proben. Primäres Ziel ist die Identifizierung von Personen mit multiplem Myelom, die ein erhöhtes Risiko für die Entwicklung einer Therapieresistenz aufweisen. Dieser strategische Schritt spiegelt das Engagement des Unternehmens wider, die Präzisionsmedizin im Zusammenhang mit multiplem Myelom voranzutreiben und potenziell zu maßgeschneiderten und effektiveren Therapieansätzen für Patienten mit Therapieresistenzen zu führen.
- Im Jahr 2022 brachte Sandoz mit Lenalidomid ein generisches Onkologiemedikament auf den Markt, das den neuesten Leitlinien der Europäischen Gesellschaft für Medizinische Onkologie (ESMO) entspricht. Dieses Medikament ist für verschiedene hämatoonkologische Erkrankungen indiziert. Es ist für Patienten in 19 europäischen Ländern verfügbar und bietet eine kostengünstige Alternative unter Einhaltung der etablierten medizinischen Standards für die in den ESMO-Leitlinien festgelegten onkologischen Behandlungen.
- Im Jahr 2022 schloss Kite, ein Gilead-Unternehmen, eine globale strategische Zusammenarbeit mit Arcellx zur gemeinsamen Weiterentwicklung und Vermarktung der T-Zelltherapie CART-ddBCMA, die für Patienten mit rezidiviertem oder refraktärem multiplem Myelom entwickelt wurde. Im Rahmen dieser Vereinbarung verpflichtete sich Arcellx zu einer Vorauszahlung von 225 Millionen US-Dollar an Kite. Die Therapie befindet sich derzeit in der klinischen Phase I und stellt einen wichtigen Schritt in der Behandlung anspruchsvoller Fälle von multiplem Myelom dar.
- Im Jahr 2022 erteilte die FDA Janssen Biotech die Zulassung für Teclistamab-cqyv, einen bahnbrechenden bispezifischen, gegen das B-Zell-Reifungsantigen (BCMA) gerichteten CD3-T-Zell-Engager. Diese Zulassung richtet sich speziell an erwachsene Patienten mit rezidiviertem oder refraktärem multiplem Myelom. Teclistamab-cqyv stellt einen bedeutenden Fortschritt in der Immuntherapie dar und bietet eine vielversprechende Therapieoption für Patienten, die mit der Behandlung dieser komplexen hämatologischen Malignität konfrontiert sind.
- Im Jahr 2021 übernahm AbbVie TeneoOne und sicherte sich damit das wichtige Immuntherapeutikum TNB-383B zur Behandlung des Multiplen Myeloms. Dieser bispezifische Antikörper zielt auf BCMA und CD3 ab und nutzt das körpereigene Immunsystem, um BCMA-exprimierende Tumorzellen zu eliminieren. Die Übernahme folgte auf eine im Februar 2019 angekündigte strategische Partnerschaft zwischen AbbVie und TeneoOne zur Entwicklung und Vermarktung von TNB-383B. AbbVie übte sein Übernahmerecht aufgrund positiver Zwischenergebnisse der Phase-I-Forschung aus.
Regionale Analyse
Geografisch betrachtet sind die im nordamerikanischen Marktbericht zur Multiple-Myelom-Diagnostik abgedeckten Länder die USA, Kanada und Mexiko.
Laut Marktforschungsanalyse von Data Bridge:
Die USA sind im Prognosezeitraum 2023–2030 das dominierende Land auf dem nordamerikanischen Markt für Multiple-Myelom-Diagnostik
Die USA dominieren den Markt für Multiple-Myelom-Diagnostik, angetrieben von günstigen Erstattungsrichtlinien. Dieses vorteilhafte Umfeld ermöglicht dem Markt lukratives Wachstum von 2023 bis 2030. Die günstigen Erstattungsrichtlinien der Region fördern Innovationen und Investitionen in diagnostische Lösungen und tragen so zu ihrer herausragenden Rolle bei der Behandlung des Multiplen Myeloms bei. Der Prognosezeitraum erwartet ein anhaltendes Wachstum und unterstreicht die Rolle der USA als Schlüsselakteur bei der Weiterentwicklung der Diagnostik dieser Erkrankung.
Für detailliertere Informationen zum nordamerikanischen Marktbericht zur Multiple-Myelom-Diagnostik klicken Sie hier – https://www.databridgemarketresearch.com/reports/north-america-multiple-myeloma-diagnostic-market