새로운 최첨단 기술의 도입 증가와 비만 인구 증가로 시장이 확대되고 있습니다. 대기업과 중소기업이 공존하는 상황에서, 여러 주요 기업의 시장은 다소 세분화되어 있습니다. Siemens Healthcare GmbH, Thermo Fisher Scientific Inc., BD와 같은 기업들이 미국 다발성 골수종 진단 시장의 주요 선두 기업으로 자리 잡으면서, 다발성 골수종 진단 시장의 경쟁은 점점 더 치열해지고 있습니다.
전체 보고서는 https://www.databridgemarketresearch.com/reports/us-multiple-myeloma-diagnostic-market 에서 확인하세요.
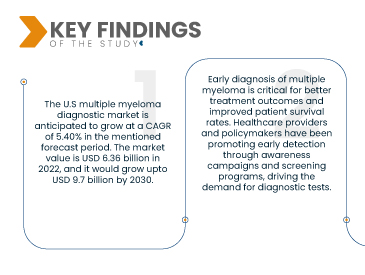
데이터 브리지 마켓 리서치(Data Bridge Market Research)는 미국 다발성 골수종 진단 시장이 2023년부터 2030년까지 연평균 성장률(CAGR) 5.40%로 성장할 것으로 분석했습니다. 시장 규모는 2022년 63억 6천만 달러에서 2030년까지 97억 달러까지 성장할 것으로 예상됩니다. 다발성 골수종은 형질세포에 영향을 미치는 혈액암의 한 유형입니다. 인구 고령화와 생활 습관의 변화는 미국에서 다발성 골수종 발병률과 유병률 증가에 기여했습니다. 발병 건수가 증가함에 따라 효과적인 진단 도구에 대한 수요 또한 증가하고 있습니다.
증가하는 의료비 지출이 시장 성장률을 견인할 것으로 예상됩니다.
미국의 의료비 지출 증가는 첨단 진단 기술과 검사의 개발 및 도입에 긍정적입니다. 이러한 자금 지원 증가는 의료 서비스 제공자들이 최첨단 진단 도구에 투자할 수 있도록 하여 더욱 정확하고 효율적인 질병 진단을 가능하게 합니다. 또한, 진단 시술에 대한 보험급여 정책 개선은 재정적 인센티브를 제공하여 의료 서비스 제공자들이 이러한 첨단 진단 솔루션을 제공하고 활용하도록 장려하고, 궁극적으로 환자 치료와 치료 결과를 향상시킵니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2023년부터 2030년까지
|
기준 연도
|
2022
|
역사적인 해
|
2021 (2015-2020년으로 맞춤 설정 가능)
|
양적 단위
|
매출(10억 달러), 볼륨(단위), 가격(USD)
|
다루는 세그먼트
|
질병(무증상(무증상) 다발성 골수종, 활동성(증상) 다발성 골수종, 골수 외 형질세포종, 경쇄 골수종, 비분비성 골수종 및 희귀 골수종), 검사 유형(혈액 검사, 소변 검사, 골수 검사 및 다발성 골수종의 유전체 시퀀싱), 유형(기기 및 소모품, 시약), 최종 사용자(병원, 전문 클리닉, 학술 및 연구 기관 및 기타), 유통 채널(소매 약국 및 약국, 병원 약국, 온라인 약국, 기타)
|
시장 참여자 포함
|
CareFusion(미국), CR Bard(미국), Straub Medical AG(미국), FlowJo LLC(미국), NeoGenomics Laboratories(미국), Helena Laboratories Corporation(미국), Bracco(이탈리아), ZYTOVISION GmbH(독일), Adaptive Biotechnologies(미국), BioVendor R&D(미국), TriVitron Healthcare(인도), Eurofins Discovery(프랑스), Quest Diagnostic Incorporated(미국)
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
미국 다발성 골수종 진단 시장은 질병, 검사 유형, 유형, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다.
- 미국의 다발성 골수종 진단 시장은 질병을 기준으로 무증상(무증상) 다발성 골수종, 활동성(증상이 있는) 다발성 골수종, 뼈의 고립성 형질세포종, 골수외 형질세포종, 경쇄 골수종, 비분비성 골수종 및 희귀 유형의 골수종으로 구분됩니다.
- 미국 다발성 골수종 진단 시장은 검사 유형을 기준으로 혈액 검사, 소변 검사, 골수 검사 및 다발성 골수종 게놈 시퀀싱 으로 세분화됩니다.
- 미국 다발성 골수종 진단 시장은 유형에 따라 장비 및 소모품, 시약으로 세분화됩니다.
- 최종 사용자를 기준으로 미국 다발성 골수종 진단 시장은 병원, 전문 클리닉, 학술 및 연구 기관 및 기타로 세분화됩니다.
- 유통 채널을 기준으로 볼 때, 미국 다발성 골수종 진단 시장은 소매 약국 및 약국, 병원 약국, 온라인 약국, 기타로 구분됩니다.
주요 플레이어
Data Bridge Market Research는 미국 다발성 골수종 진단 시장의 주요 기업으로 NeoGenomics Laboratories(미국), Helena Laboratories Corporation(미국), Bracco(이탈리아), ZYTOVISION GmbH(독일), Adaptive Biotechnologies(미국), BioVendor R&D(미국)를 꼽았습니다.
시장 개발
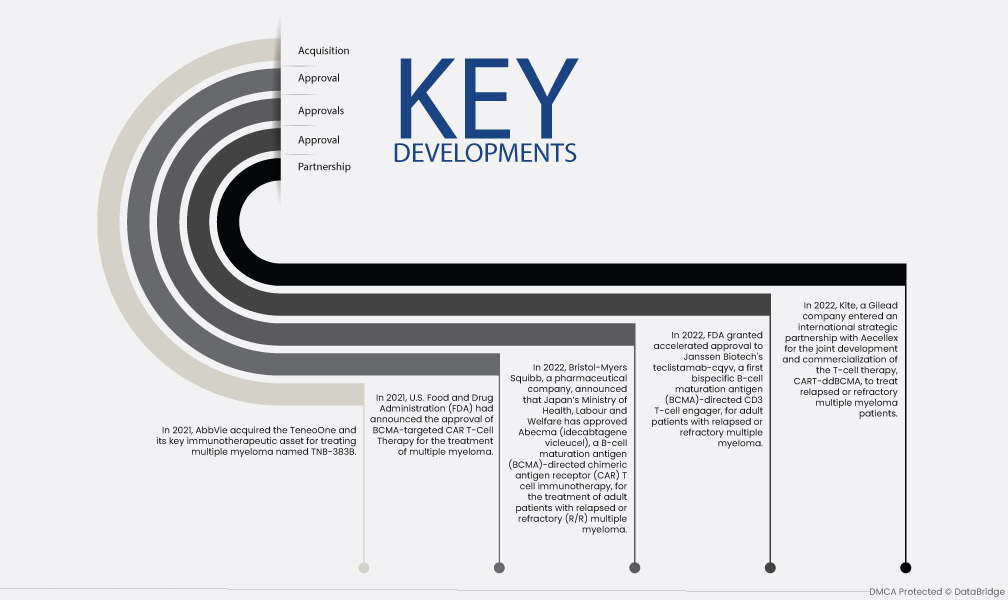
- 2022년, 길리어드 계열사인 카이트는 재발성 또는 내성성 다발성 골수종 환자를 치료하기 위한 T세포 치료제인 CART-ddBCMA의 공동 개발 및 상용화를 위해 에셀렉스와 국제 전략적 파트너십을 체결했습니다.
- 2022년 FDA는 재발성 또는 내성성 다발성 골수종 성인 환자를 대상으로 최초의 이중특이성 B세포 성숙 항원(BCMA)을 표적으로 하는 CD3 T세포 결합제인 Janssen Biotech의 teclistamab-cqyv에 대한 신속 승인을 내렸습니다.
- 제약 회사인 브리스톨-마이어스 스퀴브는 2022년에 일본 후생노동성이 재발성 또는 내성(R/R) 다발성 골수종을 앓는 성인 환자를 치료하기 위해 B세포 성숙 항원(BCMA)을 표적으로 하는 키메라 항원 수용체(CAR) T세포 면역 요법인 아베크마(Abecma, 성분명: 이데카브타진 비클레우셀)를 승인했다고 발표했습니다.
- 2021년, 미국 식품의약국(FDA)은 다발성 골수종 치료를 위한 BCMA 표적 CAR T세포 치료법의 승인을 발표했습니다.
- 2021년에 AbbVie는 다발성 골수종 치료를 위한 핵심 면역 치료 자산인 TeneoOne을 인수했으며, 이 약물의 이름은 TNB-383B입니다.
미국 다발성 골수종 진단 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요. - https://www.databridgemarketresearch.com/reports/us-multiple-myeloma-diagnostic-market