النشاط الرئيسي لصناعة الأدوية هو إدارة الجودة. يجب بيع الأدوية كتركيبات معقمة، وفعالة علاجيًا، وموثوقة، وقابلة للتنبؤ بأدائها. يجري تطوير عوامل دوائية جديدة ومحسّنة بوتيرة متسارعة. وفي الوقت نفسه، تُطوّر أساليب تحليلية أكثر دقة وتطورًا لتقييمها. ونظرًا لارتفاع معدل الإصابة بالأمراض المزمنة وظهور جائحة كوفيد-19، ازداد الطلب على الأدوية والأجهزة الطبية بشكل متناسب. ويتزايد الطلب على اختبارات السموم مع ازدياد الطلب على منتجات التكنولوجيا الحيوية والصناعات الدوائية. تُعد دراسة السموم جزءًا أساسيًا من تطوير الأدوية، إذ تُستخدم لتحديد سمية الدواء من خلال تحديد تأثيره على بنية/وظيفة الأعضاء. وتوفر الدراسة معلومات ومعارف مهمة تستخدمها الهيئات التنظيمية، من بين جهات أخرى، للوقاية من الأمراض أو تقليل احتمالية حدوثها أو غيرها من النتائج الصحية السلبية. وقد أصدرت إدارة الغذاء والدواء الأمريكية العديد من الوثائق الإرشادية للصناعة، مثل اختبارات سلامة مستقلبات الأدوية، والاستقلاب المختبري، وتفاعلات الأدوية بوساطة الناقل، ودراسات التفاعلات الدوائية السريرية.
في أبريل 2020، وفقًا لمقال نُشر في NCBI، تطور اختبار السمية على مدار العشرين عامًا الماضية، بسبب القلق المتزايد بشأن استدامة منهجيات علم السموم.
علاوة على ذلك، تُفاقم اللوائح الحكومية الصارمة المتعلقة بموافقة الأدوية والأجهزة الطبية الطلب على هذه السوق. وتزداد الحاجة إلى منتجات طبية معقمة لضمان سلامة المرضى، بالإضافة إلى حماية شركات الأدوية والأجهزة الطبية من سحب منتجاتها. لذا، يُسهم الطلب المتزايد على منتجات اختبار السموم في قطاعي الأدوية والتكنولوجيا الحيوية في نمو هذا السوق.
يمكنك الوصول إلى التقرير الكامل على @ https://www.databridgemarketresearch.com/reports/north-america-vitro-toxicology-testing-market
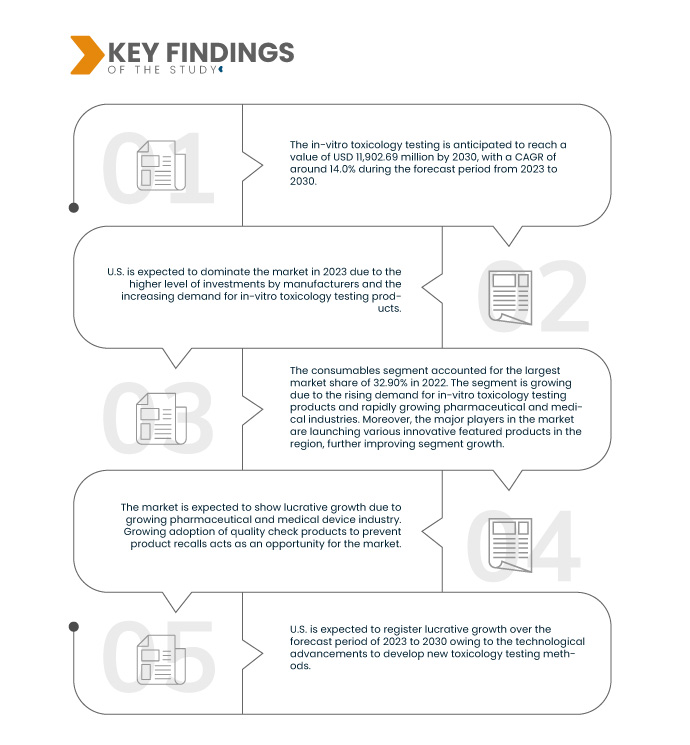
تشير تحليلات Data Bridge Market Research إلى أن سوق اختبار السموم المختبرية في أمريكا الشمالية من المتوقع أن ينمو بمعدل نمو سنوي مركب قدره 14.0٪ في الفترة المتوقعة من 2023 إلى 2030 ومن المتوقع أن يصل إلى 11،902.69 مليون دولار أمريكي بحلول عام 2030. ومن المتوقع أن تدفع صناعات الأدوية والأجهزة الطبية سريعة النمو نمو السوق.
النتائج الرئيسية للدراسة
صناعة الأدوية والأجهزة الطبية سريعة النمو
شهدت صناعة الأدوية والأجهزة الطبية تطوراتٍ واتجاهاتٍ ثوريةً عديدة ، ساهمت في تحسين الأدوية المتاحة للمرضى بشكل كبير. وشهدنا تأثير الذكاء الاصطناعي والبيانات الضخمة في تشخيص الأمراض وعلاجها.
إلى جانب قدرتها على علاج أمراضٍ لم تكن قابلةً للعلاج سابقًا، تُساعد فعالية الأدوية الصيدلانية الحيوية وحمايتها شركات الأدوية على النجاح. وتدعم فرصة النمو الصحي المستدام خط تطوير الأدوية البيولوجية الحالي. منذ عام ١٩٩٥، ازداد عدد براءات الاختراع المُقدمة في مجال التكنولوجيا الحيوية بنسبة ٢٥٪ سنويًا. يخضع حاليًا أكثر من ١٥٠٠ جزيء حيوي للتجارب السريرية، وقد تجاوز معدل نجاح الأدوية البيولوجية حتى الآن ضعف معدل نجاح الأدوية الجزيئية الصغيرة، حيث دخلت ١٣٪ من الأدوية الحيوية مرحلة الاختبار الأولى تمهيدًا لإطلاقها.
تعتمد صناعة الأدوية على اختبارات السموم المختبرية لتقييم سلامة الأدوية المرشحة ومخاطرها المحتملة خلال المراحل المبكرة من اكتشافها وتطويرها. تتيح هذه الاختبارات للباحثين تقييم آثار المواد على الخلايا والأنسجة والأعضاء، مما يوفر معلومات قيّمة حول سمية الأدوية وتفاعلاتها وآثارها الجانبية المحتملة. وتعزز الحاجة إلى تقييمات سمية دقيقة وموثوقة الطلب على اختبارات السموم المختبرية في صناعة الأدوية.
يُسهم ازدهار هذه الصناعات ونموها بشكل متناسب في زيادة الطلب على اختبارات السمية لمراقبة جودة المنتجات التي تُطوّرها. لذا، تُشكّل صناعات الأدوية والأجهزة الطبية سريعة النموّ دافعًا لنموّ السوق.
نطاق التقرير وتقسيم السوق
مقياس التقرير
|
تفاصيل
|
فترة التنبؤ
|
من 2023 إلى 2030
|
سنة الأساس
|
2022
|
السنوات التاريخية
|
2021 (قابلة للتخصيص حتى 2015 - 2020)
|
الوحدات الكمية
|
الإيرادات بالملايين من الدولارات الأمريكية
|
القطاعات المغطاة
|
المنتج والخدمة (المواد الاستهلاكية، والخدمات، والتحاليل، والمعدات، والبرامج)، ونقطة نهاية واختبار علم السموم (اختبار الامتصاص، والتوزيع، والأيض، والإخراج، واختبار السمية الخلوية، واختبار السمية الجينية، واختبار السمية الجلدية، واختبار السمية العينية، واختبار سمية الأعضاء، واختبار تهيج الجلد، والتآكل، والتحسس، واختبار السمية الضوئية، ونقاط نهاية واختبارات السمية الأخرى)، والتكنولوجيا (تقنيات زراعة الخلايا، وتقنيات الإنتاجية العالية، والتصوير الجزيئي، وتقنية OMICS)، والطريقة (التحاليل الخلوية، والتحاليل الكيميائية الحيوية، والنماذج خارج الجسم الحي، والنماذج الحاسوبية)، والصناعة (شركات الأدوية والمستحضرات الصيدلانية الحيوية، والتشخيص، والأغذية، والمواد الكيميائية ، ومستحضرات التجميل، والمنتجات المنزلية)، وقناة التوزيع (العطاء المباشر، ومبيعات التجزئة، وغيرها)
|
الدول المغطاة
|
الولايات المتحدة وكندا والمكسيك
|
الجهات الفاعلة في السوق المغطاة
|
شركة Thermo Fisher Scientific Inc. (الولايات المتحدة)، شركة Labcorp Drug Development (الولايات المتحدة)، شركة Merck KGaA (ألمانيا)، شركة Charles River Laboratories (الولايات المتحدة)، شركة Lonza (سويسرا)، شركة Bio-Rad Laboratories, Inc. (الولايات المتحدة)، شركة Catalent, Inc. (الولايات المتحدة)، شركة SGS Société Générale de Surveillance SA (سويسرا)، شركة QIAGEN (ألمانيا)، شركة Intertek Group plc. (المملكة المتحدة)، شركة Eurofins Scientific (لوكسمبورغ)، شركة Promega Corporation (الولايات المتحدة)، شركة Aragen Life Sciences Ltd. (الهند)، شركة Cyprotex Plc. (المملكة المتحدة)، شركة Shanghai Medicilon Inc. (الصين)، شركة Creative Biolabs (الولايات المتحدة)، شركة BioIVT (الولايات المتحدة)، شركة AAT Bioquest, Inc. (الولايات المتحدة)، شركة Gentronix (المملكة المتحدة)، شركة IONTOX (الولايات المتحدة)، شركة InSphero (الولايات المتحدة)، شركة MB Research Laboratories (الولايات المتحدة)، شركة Creative Bioarray (الولايات المتحدة)، وشركة Preferred Cell Systems (الولايات المتحدة).
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تتضمن أيضًا تحليلًا متعمقًا من الخبراء وعلم الأوبئة للمرضى وتحليل خطوط الأنابيب وتحليل التسعير والإطار التنظيمي.
|
تحليل القطاعات
يتم تقسيم سوق اختبارات السموم في المختبر في أمريكا الشمالية إلى ستة قطاعات بارزة مثل المنتج والخدمة ونقطة نهاية اختبار السموم والتكنولوجيا والطريقة والصناعة وقناة التوزيع.
- استنادًا إلى المنتج والخدمة، يتم تقسيم سوق اختبار السموم في المختبر في أمريكا الشمالية إلى المواد الاستهلاكية والخدمات والاختبارات والمعدات والبرمجيات.
في عام 2023، من المتوقع أن يهيمن قطاع المواد الاستهلاكية على سوق اختبارات السموم المختبرية في أمريكا الشمالية
ومن المتوقع أن يهيمن قطاع المواد الاستهلاكية على السوق في عام 2023 بحصة سوقية تبلغ 33.41% بسبب الطلب المتزايد عليه بين السكان.
- بناءً على نقاط نهاية واختبارات السمية، يُقسّم السوق إلى اختبارات ADME (الامتصاص، التوزيع، الأيض، والإخراج)، واختبارات السمية الخلوية، واختبارات السمية الجينية، واختبارات السمية الجلدية، واختبارات السمية العينية، واختبارات سمية الأعضاء، واختبارات تهيج الجلد، والتآكل، والتحسس، واختبارات السمية الضوئية، وغيرها من نقاط نهاية واختبارات السمية. في عام 2023، من المتوقع أن يهيمن قطاع اختبارات ADME (الامتصاص، التوزيع، الأيض، والإخراج) على السوق بحصة سوقية تبلغ 21.15%.
- بناءً على التكنولوجيا، يتم تقسيم السوق إلى تقنيات زراعة الخلايا، وتقنيات الإنتاجية العالية، والتصوير الجزيئي، وتقنية OMICS.
في عام 2023، من المتوقع أن تهيمن شريحة تقنيات زراعة الخلايا على سوق اختبارات السموم المختبرية في أمريكا الشمالية
ومن المتوقع أن تهيمن تقنية زراعة الخلايا على السوق في عام 2023 بحصة سوقية تبلغ 34.57% بسبب التقدم التكنولوجي المتزايد.
- بناءً على المنهجية المتبعة، يُقسّم السوق إلى فحوصات خلوية، وفحوصات كيميائية حيوية، ونماذج خارج الجسم الحي، ونماذج حاسوبية. في عام ٢٠٢٣، من المتوقع أن يهيمن قطاع الفحوصات الخلوية على السوق بحصة سوقية تبلغ ٣٧.٥٨٪.
- بناءً على القطاع، يُقسّم السوق إلى شركات الأدوية والأدوية الحيوية، وشركات التشخيص، وشركات الأغذية، وشركات الكيماويات، وشركات مستحضرات التجميل والمنتجات المنزلية. في عام 2023، من المتوقع أن تهيمن شركات الأدوية والأدوية الحيوية على السوق بحصة سوقية تبلغ 32.81%.
- بناءً على قنوات التوزيع، يُقسّم السوق إلى مناقصة مباشرة، ومبيعات التجزئة، وغيرها. وفي عام ٢٠٢٣، من المتوقع أن يهيمن قطاع المناقصة المباشرة على السوق بحصة سوقية تبلغ ٤٨.٨٧٪.
اللاعبون الرئيسيون
قامت شركة Data Bridge Market Research بتحليل شركة Thermo Fisher Scientific Inc. (الولايات المتحدة)، وLabcorp Drug Development (الولايات المتحدة)، وMerck KGaA (ألمانيا)، وCharles River Laboratories (الولايات المتحدة)، وBio-Rad Laboratories, Inc. (الولايات المتحدة)، باعتبارها اللاعبين الرئيسيين في سوق اختبارات السموم في المختبر في أمريكا الشمالية.
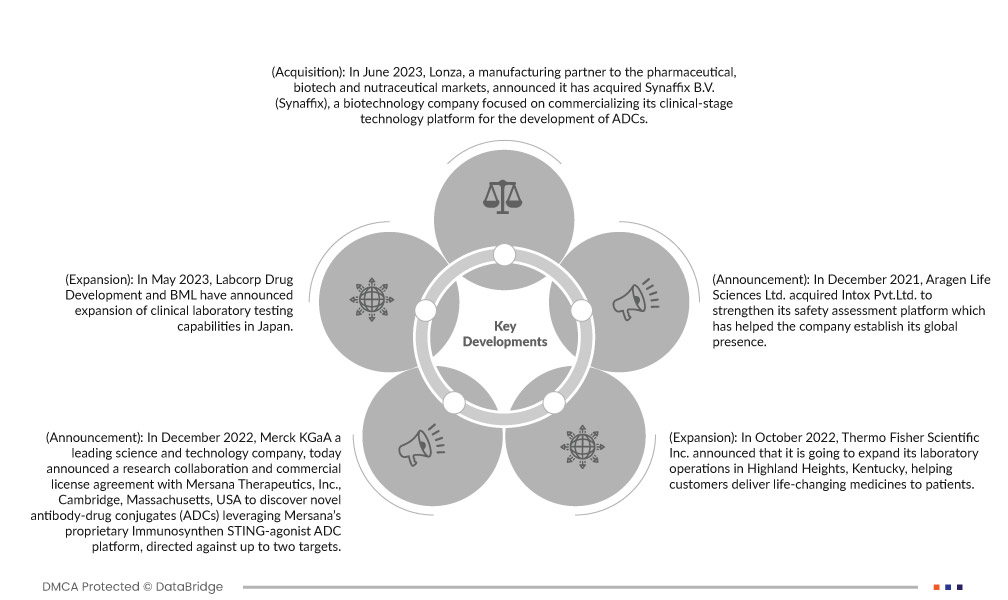
تطورات السوق
- في يونيو 2023، أعلنت شركة لونزا، الشريك التصنيعي في أمريكا الشمالية لأسواق الأدوية والتكنولوجيا الحيوية والمكملات الغذائية، عن استحواذها على شركة سينافيكس بي في (سينافيكس)، وهي شركة تكنولوجيا حيوية تُركز على تسويق منصتها التكنولوجية في المرحلة السريرية لتطوير مضادات الالتهاب اللاستيرويدية. سيساعد هذا الشركة على توسيع حضورها في أمريكا الشمالية.
- في مايو 2023، أعلنت شركة لابكورب لتطوير الأدوية وشركة بي إم إل عن توسيع قدراتها في مجال الاختبارات المعملية السريرية في اليابان. سيساعد هذا الشركة على تعزيز محفظة منتجاتها في هذه المنطقة.
- في ديسمبر 2022، أعلنت شركة Merck KGaA، وهي شركة رائدة في مجال العلوم والتكنولوجيا، اليوم عن تعاون بحثي واتفاقية ترخيص تجاري مع شركة Mersana Therapeutics، Inc.، كامبريدج، ماساتشوستس، الولايات المتحدة الأمريكية لاكتشاف مركبات دوائية مضادة جديدة (ADCs) تستفيد من منصة Immunosynthen STING-agonist ADC الخاصة بشركة Mersana، الموجهة ضد ما يصل إلى هدفين.
- في أكتوبر 2022، أعلنت شركة ثيرمو فيشر ساينتفك عن عزمها توسيع نطاق عمليات مختبرها في هايلاند هايتس، كنتاكي، لمساعدة العملاء على توفير أدوية تُحدث فرقًا إيجابيًا في حياتهم. يوفر المرفق الحالي، الذي يشمل مختبرًا مركزيًا وعمليات للمؤشرات الحيوية، لعملاء الأدوية الحيوية خدمات مختبرية عالية الجودة لتسريع تطوير الأدوية. وقد ساعد هذا الشركة على توسيع نطاق أعمالها في مجال التشخيص السريري في مناطق مختلفة حول العالم، وساهم في تعزيز حضورها في السوق الأمريكية الشمالية.
- في ديسمبر 2021، استحوذت شركة Aragen Life Sciences Ltd. على شركة Intox Pvt. Ltd. لتعزيز منصة تقييم السلامة الخاصة بها والتي ساعدت الشركة على ترسيخ وجودها.
التحليل الإقليمي
من الناحية الجغرافية، البلدان التي يغطيها سوق اختبار السموم في المختبر في أمريكا الشمالية هي الولايات المتحدة وكندا والمكسيك.
وفقًا لتحليل Data Bridge Market Research:
من المتوقع أن تهيمن الولايات المتحدة على سوق اختبارات السموم المختبرية في أمريكا الشمالية وأن تصبح الدولة الأسرع نموًا فيها.
من المتوقع أن تُهيمن الولايات المتحدة على سوق اختبارات السموم المختبرية في أمريكا الشمالية، بفضل ارتفاع مستوى استثمارات مختلف الشركات المصنعة، وتزايد الطلب على هذه الاختبارات في البلاد. ويُقدّر أن تكون الولايات المتحدة أسرع الدول نموًا في سوق اختبارات السموم المختبرية في أمريكا الشمالية، بفضل تزايد اعتماد التكنولوجيا المتقدمة وإطلاق منتجات جديدة في هذه المنطقة.
لمزيد من المعلومات التفصيلية حول تقرير سوق اختبارات السموم المختبرية في أمريكا الشمالية ، انقر هنا - https://www.databridgemarketresearch.com/reports/north-america-vitro-toxicology-testing-market