1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
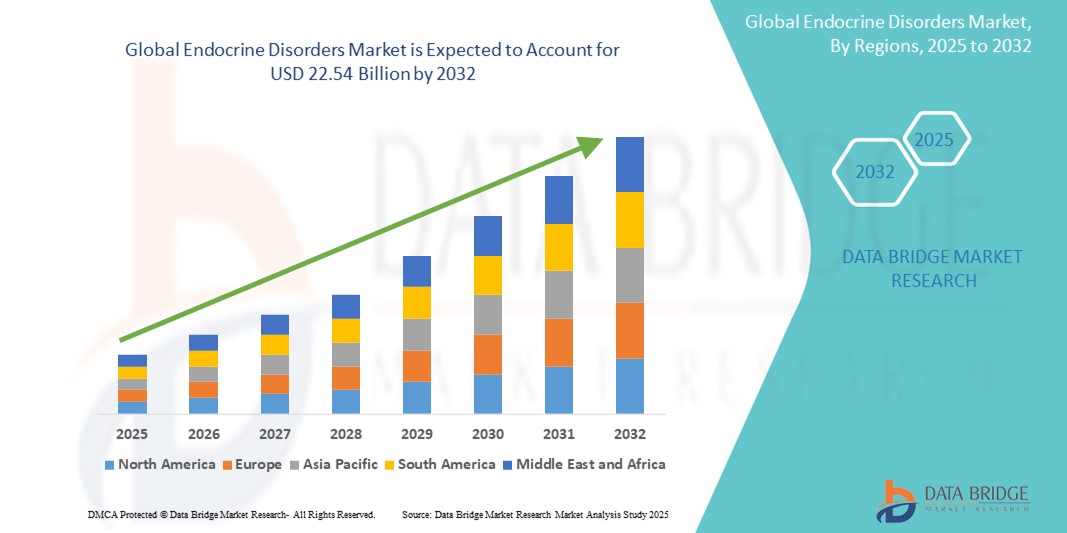
1.3 OVERVIEW OF GLOBAL ENDOCRINE DISORDERS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ENDOCRINE DISORDERS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ENDOCRINE DISORDERS MARKET : RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL ENDOCRINE DISORDERS MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL ENDOCRINE DISORDERS MARKET , BY DISORDER TYPE
16.1 OVERVIEW
16.2 DIABETES
16.2.1 TYPE 1 DIABETES
16.2.2 TYPE 2 DIABETES
16.2.3 GESTATIONAL DIABETES
16.2.4 PRE-DIABETES
16.3 THYROID DISORDERS
16.3.1 1.2.1 HYPOTHYROIDISM
16.3.2 1.2.2 HYPERTHYROIDISM
16.3.3 1.2.3 THYROID CANCER
16.3.4 1.2.4 THYROID NODULES
16.3.5 GOITER
16.4 ADRENAL DISORDERS
16.4.1 ADDISON'S DISEASE
16.4.2 CUSHING'S SYNDROME
16.4.3 ADRENAL INSUFFICIENCY
16.4.4 ADRENAL HYPERPLASIA
16.5 PITUITARY DISORDERS
16.5.1 PITUITARY TUMORS
16.5.2 HYPOPITUITARISM
16.5.3 HYPERPROLACTINEMIA
16.5.4 ACROMEGALY
16.6 PARATHYROID DISORDERS
16.6.1 HYPERPARATHYROIDISM
16.6.2 HYPOPARATHYROIDISM
16.6.3 PARATHYROID CANCER
16.7 OTHER
17 GLOBAL ENDOCRINE DISORDERS MARKET , BY TREATMENT TYPE
17.1 OVERVIEW
17.2 DRUG THERAPY
17.2.1 BY TYPE
17.2.1.1. HORMONE REPLACEMENT THERAPY (HRT)
17.2.1.1.1. ESTROGEN
17.2.1.1.2. TESTOSTERONE
17.2.1.1.3. GROWTH HORMONE
17.2.1.1.4. PROGESTERONE REPLACEMENT
17.2.1.1.5. OTHERS
17.2.1.2. INSULIN THERAPY
17.2.1.2.1. RAPID-ACTING INSULIN
17.2.1.2.2. LONG-ACTING INSULIN
17.2.1.2.3. INTERMEDIATE-ACTING INSULIN
17.2.1.3. CORTICOSTEROIDS
17.2.1.4. ANTI-THYROID MEDICATIONS
17.2.1.5. HYPOGLYCEMICS
17.2.1.6. OTHERS
17.2.2 BY ROUTE OF ADMINISTRATION
17.2.2.1. ORAL
17.2.2.2. PARENTERAL
17.2.2.3. OTHERS
17.2.3 BY DRUG CLASS
17.2.3.1. BRANDED
17.2.3.2. GENERIC
17.3 SURGERY
17.3.1 THYROIDECTOMY
17.3.1.1. PARTIAL THYROIDECTOMY
17.3.1.2. TOTAL THYROIDECTOMY
17.3.2 ADRENALECTOMY
17.3.2.1. LAPAROSCOPIC ADRENALECTOMY
17.3.2.2. OPEN ADRENALECTOMY
17.3.3 PARATHYROIDECTOMY
17.3.4 PITUITARY SURGERY
17.3.4.1. TRANSSPHENOIDAL SURGERY
17.3.4.2. CRANIOTOMY
17.4 RADIOTHERAPY
17.4.1 RADIOACTIVE IODINE THERAPY
17.4.2 STEREOTACTIC RADIOSURGERY
17.5 OTHERS
18 GLOBAL ENDOCRINE DISORDERS MARKET , BY DIAGNOSTIC TYPE
18.1 OVERVIEW
18.2 BLOOD TESTS
18.2.1 HORMONE LEVEL TESTS
18.2.2 GLUCOSE TESTS
18.2.3 ANTIBODY TESTS
18.2.4 GENETIC TESTING
18.3 IMAGING TESTS
18.3.1 ULTRASOUND
18.3.1.1. HYPOTHYROIDISM THYROID ULTRASOUND
18.3.1.2. ADRENAL GLAND ULTRASOUND
18.3.2 MRI
18.3.3 CT SCAN
18.3.4 OTHERS
18.4 BIOPSY
18.4.1 CORE NEEDLE BIOPSY
18.4.2 FINE NEEDLE ASPIRATION BIOPSY (FNAB)
18.5 FUNCTIONAL TESTS
18.6 OTHERS
19 GLOBAL ENDOCRINE DISORDERS MARKET , BY PATIENT AGE GROUP
19.1 OVERVIEW
19.2 PEDIATRICS
19.3 ADULTS
19.4 GERIARTIC
20 GLOBAL ENDOCRINE DISORDERS MARKET , BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 SPECIALITY CLINICS
20.4 HOMECARE
20.5 ACADEMIC AND RESEARCH INSTITUTES
20.6 OTHERS
21 GLOBAL ENDOCRINE DISORDERS MARKET , BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDERS
21.3 RETAIL SALES
21.3.1 OFFLINE PHARMACIES
21.3.2 ONLINE PHARMACIES
21.4 OTHERS
22 GLOBAL ENDOCRINE DISORDERS MARKET , BY GEOGRAPHY
GLOBAL ENDOCRINE DISORDERS MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.1 NORTH AMERICA
22.1.1 U.S.
22.1.2 CANADA
22.1.3 MEXICO
22.2 EUROPE
22.2.1 GERMANY
22.2.2 FRANCE
22.2.3 U.K.
22.2.4 ITALY
22.2.5 SPAIN
22.2.6 RUSSIA
22.2.7 TURKEY
22.2.8 BELGIUM
22.2.9 NETHERLANDS
22.2.10 SWITZERLAND
22.2.11 REST OF EUROPE
22.3 ASIA-PACIFIC
22.3.1 JAPAN
22.3.2 CHINA
22.3.3 SOUTH KOREA
22.3.4 INDIA
22.3.5 AUSTRALIA
22.3.6 SINGAPORE
22.3.7 THAILAND
22.3.8 MALAYSIA
22.3.9 INDONESIA
22.3.10 PHILIPPINES
22.3.11 REST OF ASIA-PACIFIC
22.4 SOUTH AMERICA
22.4.1 BRAZIL
22.4.2 ARGENTINA
22.4.3 PERU
22.4.4 CHILE
22.4.5 COLOMBIA
22.4.6 VENEZUELA
22.4.7 REST OF SOUTH AMERICA
22.5 MIDDLE EAST AND AFRICA
22.5.1 SOUTH AFRICA
22.5.2 SAUDI ARABIA
22.5.3 UAE
22.5.4 EGYPT
22.5.5 ISRAEL
22.5.6 REST OF MIDDLE EAST AND AFRICA
22.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL ENDOCRINE DISORDERS MARKET , COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL ENDOCRINE DISORDERS MARKET , SWOT AND DBMR ANALYSIS
25 GLOBAL ENDOCRINE DISORDERS MARKET , COMPANY PROFILE
25.1 NOVO NORDISK
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 SANOFI
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 ABBVIE
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 MERCK & CO., INC.
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 PFIZER
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 BOEHRINGER INGELHEIM
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 ROCHE
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 NOVARTIS
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 ABBOTT LABORATORIES
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPEMENTS
25.1 BRISTOL-MYERS SQUIBB
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 FRESENIUS KABI
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 IPSEN
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 HORIZON THERAPEUTICS
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 TAKEDA PHARMACEUTICAL COMPANY
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 ASCENDIS PHARMA A/S
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 BIO-RAD LABORATORIES, INC.
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 AUROBINDO PHARMA
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 JOHNSON & JOHNSON SERVICES, INC.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 ENDO INTERNATIONAL
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
26 CONCLUSION
27 QUESTIONNAIRE
28 ABOUT DATA BRIDGE MARKET RESEARCH