Rapid Diagnostic Tests (RDT) refer to simple, fast, point-of-care diagnostic assays designed to detect specific antigens, antibodies, or biomarkers associated with diseases, typically delivering results within 10–30 minutes without the need for complex laboratory equipment. RDTs are widely used for the diagnosis of infectious diseases such as COVID-19, malaria, influenza, HIV, and dengue, as well as certain non-communicable conditions. They are commonly based on lateral flow immunoassay or similar technologies and are valued for their ease of use, portability, low cost, and suitability for decentralized or resource-limited settings. Additionally, government-led screening programs, expanding public–private healthcare partnerships, and increasing investment in diagnostic manufacturing capacity across countries such as China, India, and Southeast Asia are strengthening market penetration. However, challenges including variable regulatory standards, sensitivity limitations of certain RDTs, and inconsistent reimbursement frameworks persist across emerging markets. Overall, the APAC RDT market is evolving toward scalable, affordable, and rapid diagnostic solutions that support large-scale screening, outbreak preparedness, and improved healthcare access across diverse care settings.
Access Full Report @ https://www.databridgemarketresearch.com/reports/asia-pacific-rapid-diagnostic-tests-rdt-market
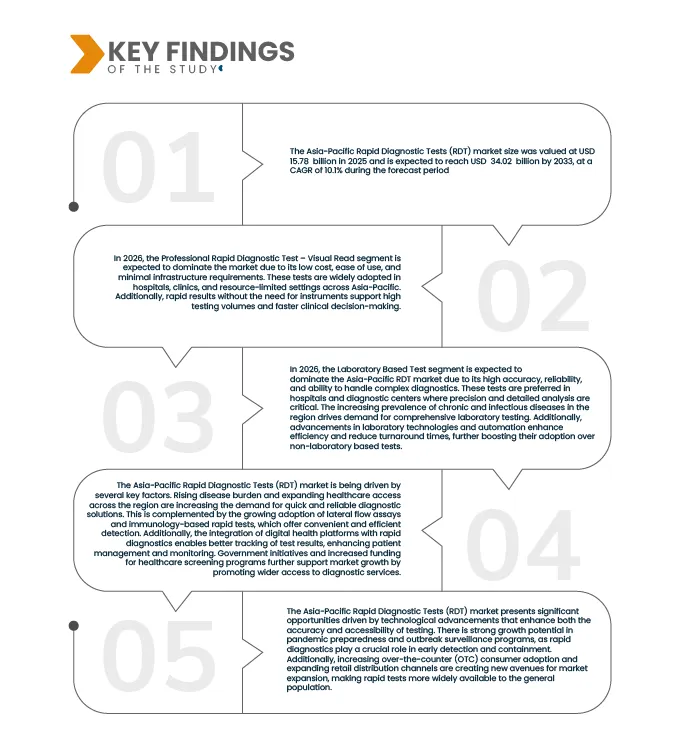
Data Bridge Market Research analyzes that the Asia-Pacific Rapid Diagnostic Tests (RDT) Market is expected to reach USD 34.02 billion by 2033 from USD 15.78 billion in 2025, growing at a substantial CAGR of 10.1% in the forecast period of 2026 to 2033.
Key Findings of the Study
Rising Disease Burden and Expanding Healthcare Access
Rising disease burden and expanding healthcare access are high and growing prevalence of infectious diseases such as dengue, malaria, tuberculosis, and COVID-19, alongside a steady increase in chronic conditions like diabetes and cardiovascular diseases. This creates strong demand for fast, accurate, and affordable diagnostic solutions. At the same time, governments across Asia-Pacific are investing in healthcare infrastructure, primary care facilities, and universal health coverage programs, especially in rural and underserved areas. RDTs are well suited to these settings due to their low cost, minimal equipment needs, and quick results, accelerating their adoption across hospitals, clinics, and community health programs.
The rising prevalence of infectious and chronic diseases in Asia‑Pacific is driving strong demand for rapid diagnostic tests (RDTs). Governments’ investments in healthcare infrastructure, primary care, and universal health coverage, especially in rural and underserved areas, are expanding access to diagnostics. Due to their low cost, ease of use, and quick results, RDTs are increasingly adopted across hospitals, clinics, and community health programs, making them a key tool in addressing the region’s growing disease burden
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2026 to 2033
|
|
Base Year
|
2025
|
|
Historic Year
|
2024 (Customizable 2018-2024)
|
|
Quantitative Units
|
Revenue in USD Billiion
|
|
Segments Covered
|
By Mode (Professional Rapid Diagnostic Test – Visual Read, Otc (Over-The-Counter) Rapid Diagnostic Test, Professional Rapid Diagnostic Test – Instrument Read, Professional Rapid Molecular Diagnostic Test – Instrument Read), By Modality (Laboratory Based Test, Non-Laboratory Based Test), By Age Group (Adult, Pediatrics), By Diagnostic Approach (In-Vitro Diagnostic (IVD) – Immunoassay / Lateral Flow, Molecular Diagnostic (MD)), By Application Area (Infectious Disease Testing, Cardiology Testing, Pregnancy & Fertility Testing, Drugs of Abuse Testing, Oncology Testing, Coagulation Testing, Others), By Price Range (Economy, Mid-Range, Premium), By End User (Hospitals & Clinics, Diagnostic Laboratories, Home Care Settings, Research & Academic Institutes, Others), By Business Type (Government Sector, Private Sector), By Country (China, Japan, India, South Korea, Australia, Indonesia, Thailand, Singapore, Malaysia, Philippines, Vietnam, Pakistan, Bangladesh, Sri Lanka, Nepal, Afghanistan, Maldives, Bhutan, Rest of Asia-Pacific).
|
|
Region Covered
|
Asia-Pacific
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd, Abbott, Sysmex Corporation, Danaher Corporation, Thermo Fisher Scientific Inc., Meril Diagnostics, Siemens Healthcare Private Limited , Bio-Rad Laboratories, Inc, BD, Werfen , QIAGEN, Wondfo , QuidelOrtho Corporation, BIOMÉRIEUX, FUJIREBIO, ACCESS BIO, SD Biosensor, INC, Trinity Biotech, J. Mitra & Co. Pvt. Ltd., Chembio Diagnostics, Inc., GENBODY INC., Precision Biomed Pvt Ltd, BIOGENIX INC. PVT. LTD, NM Medical, Nueclear, Cobalt Health, DMS Health, Cardiac Imaging, Inc., Alliance-HNI Health Care Services and, among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
Asia-Pacific Rapid Diagnostic Tests (RDT) market is categorized into eight notable segments which are based on mode, modality, age group, diagnostic approach, application area, price range, end user, business type.
- On the basis of Mode, Asia-Pacific Rapid Diagnostic Tests (RDT) Market is segmented into Professional Rapid Diagnostic Test – Visual Read, Otc (Over-The-Counter) Rapid Diagnostic Test, Professional Rapid Diagnostic Test – Instrument Read, Professional Rapid Molecular Diagnostic Test – Instrument Read.
In 2026, the Professional Rapid Diagnostic Test – Visual Read segment is expected to dominate the market
In 2026, the Professional Rapid Diagnostic Test – Visual Read segment is expected to dominate with 42.92% due to its low cost, ease of use, and minimal infrastructure requirements. These tests are widely adopted in hospitals, clinics, and resource-limited settings across Asia-Pacific. Additionally, rapid results without the need for instruments support high testing volumes and faster clinical decision-making.
- On the basis of modality, the Asia-Pacific Rapid Diagnostic Tests (RDT) market is segmented into Laboratory Based Tests and Non-Laboratory Based Tests.
In 2026, the Laboratory Based Tests segment is expected to dominate the market
In 2026, the Laboratory Based Tests segment is expected to dominate with 62.83% market share due to its high accuracy, reliability, and ability to handle complex diagnostics. These tests are preferred in hospitals and diagnostic centers where precision and detailed analysis are critical. The increasing prevalence of chronic and infectious diseases in the region drives demand for comprehensive laboratory testing. Additionally, advancements in laboratory technologies and automation enhance efficiency and reduce turnaround times, further boosting their adoption over non-laboratory based tests.
- On the basis of Age Group, the Asia-Pacific Rapid Diagnostic Tests (RDT) Market is segmented into Adult, Pediatrics. In 2026, the adult segment is expected to dominate with 80.76% market share
- On the basis of diagnostic approach, the Asia-Pacific Rapid Diagnostic Tests (RDT) market is segmented into In-Vitro Diagnostic (IVD) – Immunoassay / Lateral Flow and Molecular Diagnostic (MD). The IVD – Immunoassay / Lateral Flow segment is further segmented into Lateral Flow Immunoassay (LFIA), Visual Read RDTs (Cassette/Strip Form), Colorimetric, and Others. The Molecular Diagnostic (MD) segment is further segmented into NAAT-based Rapid, Rapid PCR, Isothermal Amplification, and Others. In 2026, In-Vitro Diagnostic (IVD) – Immunoassay / Lateral Flow segment is expected to dominate with 76.28% market share.
- On the basis of Application Area, the Asia-Pacific Rapid Diagnostic Tests (RDT) market is segmented into Infectious Disease Testing, Cardiology Testing, Pregnancy & Fertility Testing, Drugs of Abuse Testing, Oncology Testing, Coagulation Testing, and Others. The Infectious Disease Testing segment is further categorized into Respiratory Infection Tests (including Influenza A+B / A+B+C, RSV, Strep A, Mycoplasma, S. Pneumonia, Legionella, and Others), Hepatitis, HIV, Malaria, Dengue, Sexually Transmitted Diseases (Chlamydia, Gonorrhea, Syphilis, and Others), Gastrointestinal Infections (H. Pylori Ab/Ag, Typhoid, Rotavirus, C. difficile, E. coli, Norovirus, Campylobacter, Adenovirus, E71, and Others), Chikungunya, and Others. The Cardiology Testing segment includes HS-Troponin I, BNP / NT-proBNP, D-Dimer, S-Troponin T, CK-MB, HSCRP, Myoglobin, PCT, ST2, Homocysteine, and Galectin-3. The Pregnancy & Fertility Testing segment comprises HCG, LH, and Combined Fertility Panels. The Drugs of Abuse Testing segment includes Multi-panel Urine Tests and Oral Fluid Drug Tests. The Oncology Testing segment covers AFP, CEA, PSA, CA19-9, CA125, CA15-3, and Others, while the Coagulation Testing segment includes PT, PTT, ACT, FIB, and TT, with additional diagnostics grouped under Others. In 2026, Infectious Disease Testing segment is expected to dominate with 43.80% market share
- On the basis of Price Range, the Asia-Pacific Rapid Diagnostic Tests (RDT) Market is segmented into Economy, Mid-Range, Premium. In 2026, the Economy segment is expected to dominate the with 53.25% market share
- On the basis of End User, the Asia-Pacific Rapid Diagnostic Tests (RDT) market is segmented into Hospitals & Clinics, Diagnostic Laboratories, Home Care Settings, Research & Academic Institutes, and Others. The Hospitals & Clinics segment is further categorized into Public and Private hospitals and clinics. In 2026, the Hospitals segment is expected to dominate the with 49.90% market share
- On the basis of Business Type, the Asia-Pacific Rapid Diagnostic Tests (RDT) Market is segmented into Government Sector, Private Sector. In 2026, the Government Sector segment is expected to dominate the with 57.48% market share
Major Players
Data Bridge Market Research analyzes F. Hoffmann-La Roche Ltd, Abbott, Sysmex Corporation, Danaher Corporation, Thermo Fisher Scientific Inc as the major market players of the market.
Market Development
- In December 2024, Roche announced the CE Mark approval of its cobas BV/CV PCR assay, designed to accurately detect bacteria causing bacterial vaginosis and yeast linked to candida vaginitis. The test uses a single vaginal swab to deliver faster and more precise results compared to traditional diagnostic methods, which often lack accuracy and delay treatment. By enabling targeted therapies, the assay helps reduce complications and improve care for millions of women affected annually. The launch strengthens Roche Diagnostics’ sexual health portfolio, expands testing capabilities on cobas systems, and supports revenue growth through faster, efficient, and accurate molecular diagnostics.
- In November 2025, Abbott Fast diagnosis as the strongest defense against pneumonia. Pneumonia continues to be a leading infectious cause of death worldwide, especially among children, older adults, and high-risk populations. Delays in diagnosis can lead to severe complications, longer recovery times, and increased antibiotic resistance. Abbott’s rapid urine-based antigen tests enable detection of key pathogens such as Streptococcus pneumoniae and Legionella pneumophila within minutes, supporting timely and targeted treatment. These connected diagnostic solutions help clinicians provide faster, more accurate care, reduce hospital stays, minimize unnecessary antibiotic use, and improve patient outcomes globally
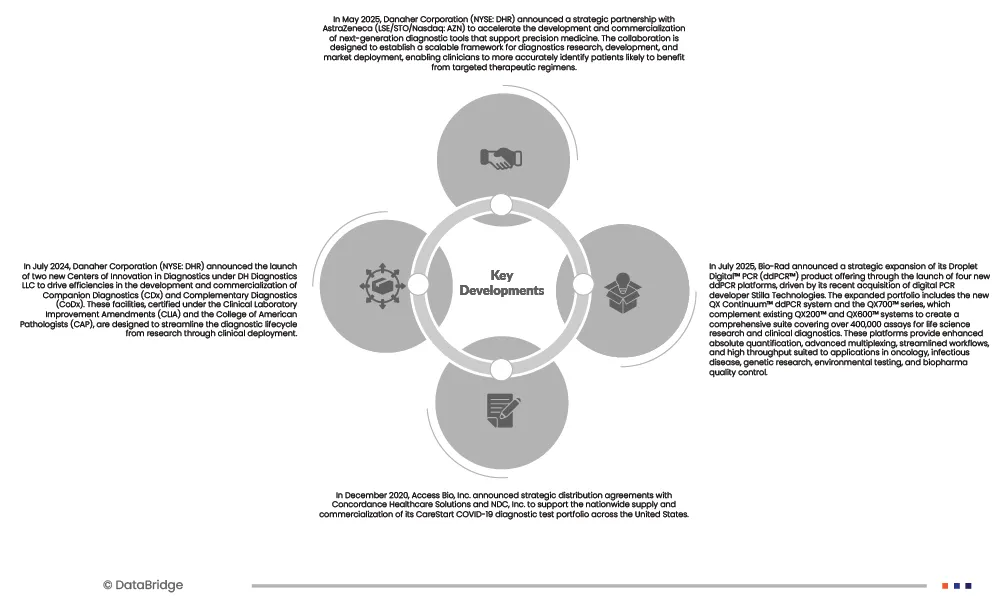
- On May 2025, Danaher Corporation (NYSE: DHR) announced a strategic partnership with AstraZeneca (LSE/STO/Nasdaq: AZN) to accelerate the development and commercialization of next-generation diagnostic tools that support precision medicine. The collaboration is designed to establish a scalable framework for diagnostics research, development, and market deployment, enabling clinicians to more accurately identify patients likely to benefit from targeted therapeutic regimens.
- In December 2020, Access Bio, Inc. announced strategic distribution agreements with Concordance Healthcare Solutions and NDC, Inc. to support the nationwide supply and commercialization of its CareStart™ COVID‑19 diagnostic test portfolio across the United States.
- In October 2025, Bio-Rad extended its partnership with Gencurix through a strategic agreement under which Bio-Rad became the exclusive distributor of Gencurix’s CE-IVD droplet digital PCR (ddPCR) oncology kits across Europe. This development strengthens Bio-Rad’s oncology diagnostics portfolio by expanding access to highly sensitive ddPCR-based assays compatible with its QXDx™ systems, supporting precise mutation detection in solid tumors and liquid biopsy applications for European clinical laboratories.
For more detailed information about the Asia-Pacific Rapid Diagnostic Tests (RDT) Market report, click here – https://www.databridgemarketresearch.com/reports/asia-pacific-rapid-diagnostic-tests-rdt-market