OVERVIEW
In early 2020, a new virus has begun to make headlines around the world due to its unprecedented speed of transmission. Its origins can be traced back to the food market in Wuhan, China in December 2019. From there, it has reached countries as far away as the United States and the Philippines. The virus (formally called SARS-CoV-2) is responsible for millions of infections worldwide and causes hundreds and thousands of deaths. The United States is the most affected country. The disease caused by infection with SARS-CoV-2 is called COVID-19, which stands for coronavirus disease 2019. As definitive treatments for confirmed COVID-19 have not yet been identified, there is significant interest in repeating existing antiviral drugs for use against COVID-19.
Several therapies have been evaluated for the treatment of COVID-19, but they have not yet been shown to be effective. Remdesivir (GS-5734), a viral RNA-dependent RNA polymerase inhibitor with inhibitory activity against SARS-CoV and respiratory syndrome in the Middle East (MERS-CoV), was early identified as a promising therapist for COVID-19 due to its ability to inhibit CoV- SARS 2 in vitro. In addition, in studies with non-human primates, remdesivir started, starting 12 hours after MERS-CoV inoculation, lung virus levels and lung damage.
IMPACT ON PRICE
A large number of challenges are being faced by the various market players due to the coming of the novel coronavirus, one such issue is the uncertainty surrounding the impact of COVID-19 on remdesivir demands. Due to the increased demand of remdesivir for coronavirus treatment and stiff competition between the market players, the price of remdesivir has decreased.
Remdesivir is expected to be in high demand as one of the treatments to date that converts COVID-19. After the intravenous drug helped shorten the hospital recovery time in a clinical trial, it was cleared of emergency use in the United States and fully approved in Japan.
The drug is thought to be more effective in treating patients earlier in the course of the disease than dexamethasone, which has reduced the number of deaths in patients in need of oxygen support and those in ventilators. However, remdesivir, in its current formulation, is only used in patients who are sick enough to require hospitalization for a five-day course. For the U.S. patients with employment insurance, Gilead said it would charge USD 3,120 for each course or USD 520 for each vial. That's a 33% increase from USD 390 per vial that Gilead said it would charge the governments of developed countries and the U.S. patients in government health programs.
All India Drugs Action Network, NGOs, have urged the Ministry of Health to price COVID-19 related experimental treatments including remdesivir, a drug first developed to treat Ebola. The Department of Pharmaceuticals had received complaints that remdesivir was being prescribed indirectly and had asked the Ministry of Health and the drug regulatory authority to monitor whether hospitals were using remdesivir instead of lower-priced drugs.
At the same time, ironically in India, the price war has begun among the general suppliers of the drug and even prices are expected to fall more as more companies start operating stocks. Zydus is the fifth Indian company to launch the drug to treat coronavirus disease by Hetero Labs, Cipla, Mylan NV and Jubilant Life Sciences. Cipla's Cipremi costs Rs 4,000, Hetero Healthcare's Covifor Rs 5,400, Mylan's version Rs 4,800 and Jubilant's JUBI-R Rs 4,700. Ahmedabad-based Zydus Cadila has launched the cheapest Remdesivir syringe for COVID-19 treatment in India to date and has launched a price war with other companies licensed by Gilead to sell it in India and other parts of the country.
With the increasing demand and increasing sale of remdesivir drugs, the competition between the remdesivir drugs manufacturers has increased market, leads to the fall of remdesivir drugs price.
IMPACT ON DEMAND
The Corona virus zone is the result of various markets around the world. This is the cause of the widespread closures and isolation that are affecting world economic activity.
Remdesivir still under research and possible treatment for COVID-19, are in high demand. Physicians are increasingly prescribing it too moderately to seriously ill patients, even though scientific evidence on their efficacy is awaited. COVID-19 outbreaks have led to a sharp increase in the demand for remdesivir and to cilizumab - two drugs that have become necessary to treat severe cases of infection. To meet demand, the Maharashtra Food and Drug Administration (FDA) plans to buy remdesivir from a company in Gujarat. With the state registering more than 7,000 new cases of COVID-19 daily, there has been a growing demand for remdesivir and tocilizumab, which have been effective in many serious cases of infection. However, this has also led to a shortage of these drugs in the market as well as an overload of some retailers.
Chemists and pharmacists are in the high demand for the antiviral drug remdesivir. The EMA's Human Rights Committee has recommended that the Gilead Sciences antiretroviral drug, remdesivir, be approved for the treatment of patients with COVID-19 in the EU, making it the first coronary antiviral drug to reach that milestone.
Figure1: Remdesivir for Treatment of COVID-19-Combination of Pulmonary and IV Administration May offer additional Benefit
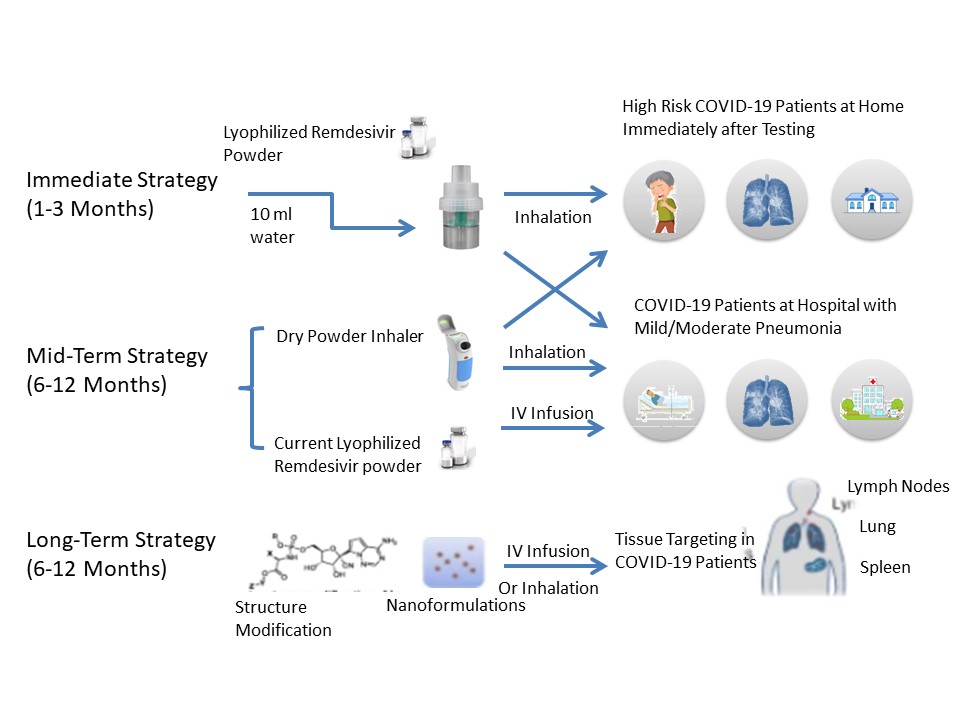
Source: Remdesivir for Treatment of COVID-19: Combination of Pulmonary and IV Administration May Offer Additional Benefit
TABLE 1. Registered Remdesivir Trials for SARS-CoV-2/COVID-19
|
study identifier
|
Study Title
|
Start Date
|
Status
|
Sponsor
|
Interventions
|
Phase
|
Study Design
|
Expected Completion Date
|
Location (s)
|
|
NCT04280705b
|
Adaptive COVID-19 Treatment Trial (ACTT)
|
February 21, 2020
|
Recruiting
|
National Institute of Allergy and Infectious Diseases (NIAID)
|
Remdesivir; remdesivir placebo
|
3
|
Clinical trial, randomized parallel assignment
|
Double (participant, investigator)
|
Treatment
|
|
ISRCTN83971151c, NCT04330690
|
Public Health Emergency SOLIDARITY Trial of Treatments for COVID- 19 Infection in Hospitalized Patients
|
March 1, 2020
|
Available
|
World Health Organization
|
Remdesivir; lopinavir/ritonavir; lopinavir/ritonavir, interferon β-1a; hydroxychloroquine; standard of care
|
3
|
Clinical trial, randomized
|
None (open label)
|
Treatment
|
|
NCT04292899b
|
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
|
March 6, 2020
|
Recruiting
|
Gilead Sciences
|
Remdesivir; Standard of Care
|
3
|
Clinical trial, randomized parallel assignment
|
None (open label)
|
Treatment
|
|
NCT04292730b study
|
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment
|
March 15, 2020
|
Recruiting
|
Gilead Sciences
|
Remdesivir; standard of care
|
3
|
Clinical trial, randomized parallel assignment
|
None (open label)
|
Treatment
|
|
study identifier
|
Study tItle
|
Start Date
|
Status
|
Sponsor
|
Interventions
|
Phase
|
Study design
|
Expected completion date
|
Location (s)
|
|
NCT04280705b
|
Adaptive COVID-19 Treatment Trial (ACTT)
|
February 21, 2020
|
Recruiting
|
National Institute of Allergy and Infectious Diseases (NIAID)
|
Remdesivir; remdesivir placebo
|
3
|
Clinical trial, randomized parallel assignment
|
Double (participant, investigator)
|
Treatment
|
|
ISRCTN83971151c, NCT04330690
|
Public Health Emergency SOLIDARITY Trial of Treatments for COVID- 19 Infection in Hospitalized Patients
|
March 1, 2020
|
Available
|
World Health Organization
|
Remdesivir; lopinavir/ritonavir; lopinavir/ritonavir, interferon β-1a; hydroxychloroquine; standard of care
|
3
|
Clinical trial, randomized
|
None (open label)
|
Treatment
|
|
NCT04292899b
|
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
|
March 6, 2020
|
Recruiting
|
Gilead Sciences
|
Remdesivir; Standard of Care
|
3
|
Clinical trial, randomized parallel assignment
|
none (open label)
|
Treatment
|
|
NCT04292730b study
|
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment
|
March 15, 2020
|
Recruiting
|
Gilead Sciences
|
Remdesivir; standard of care
|
3
|
Clinical trial, randomized parallel assignment
|
None (open label)
|
Treatment
|
|
NCT04314817b
|
Adverse Events Related to Treatments Used Against Coronavirus Disease 2019
|
March 17, 2020
|
Recruiting
|
Groupe Hospitalier Pitie-Salpetriere,CMC Ambroise Paré
|
Any drug used to treat COVID-19
|
|
Observational model, case-only
|
|
|
Source: Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19
The impact of COVID-19 has created an opportunity for the number of patients as there is high chance of adverse health effects of COVID-19 on the people.
IMPACT ON SUPPLY
As the epidemic intensifies, supply chains can be at significant risk due to over-located locations that can potentially be disrupted. The supply chain of drugs has been disrupted. The spread of COVID-19 makes it difficult for governments to use these drugs; the availability of these systems faces constant challenges due to their components of use as well as limited initial needs.
The trade restrictions have chosen nothing more than to produce the necessary medicines domestically. During the pandemic, when the demand of this drug increased, the counterfeiting and prices of imported goods also increased. This signifies that even during the COVID-19 pandemic the market players are able to maintain the supply chain.
STRATEGIC DECISIONS OF GOVERNMENT AND MANUFACTURERS
As the coronavirus continues to spread to various countries, concerns are growing about disruptions in drug production and distribution. Collaborations, agreements, initiatives of market participants such as Zydus Cadila, Gilead Sciences, Inc. market players in the pharmaceutical market have helped them expand their market. This in turn will help to increase the demand for the product among the consumers and thus increases the future sales of the company.
Market players have already taken different initiatives to combat the corona virus.
For instance,
- In August 2020, the pharmaceutical company Zydus Cadila announced that it had launched Remdesivir under the brand name Remdac, used to treat patients suffering from severe symptoms of COVID-19, in the Indian market. Zydus Cadila, priced at £ 2,800 per 100 mg vial, is the most cost-effective Remdesivir brand in India, according to Zydus Cadila. The company has also announced the drug would be made available across India through the group's strong distribution chain to government and private hospitals that treat COVID-19 patients.
- In August 2020, Pfizer Inc. announced a four-year deal with Gilead Sciences, Inc. on the Production and Delivery of Anti-Virus Trials Gilead, as one of a number of external manufacturing organizations supporting efforts to expand the range of COVID-19 research therapies. Under the terms of the agreement, Pfizer will provide contract services in McPherson, Kansas, Pfizer's facility to produce and deliver delivery for Gilead.
- In August 2020, Jubilant Generics Ltd. has installed remdesivir for injections under the brand name "JUBI-R" in India for the treatment of COVID-19. Jubilant Generics Ltd., a subsidiary of the integrated global pharmaceutical and life sciences company Jubilant Life Sciences Ltd., announced company would make the drug available to more than 1,000 COVID-19 hospitals in India.
- In June 2020, Cipla Ltd announced the launch of a generic version of remdesivir, which has been authorized for emergency treatment of COVID-19 patients by the USFDA, under the Cipremi brand. The USFDA had issued an emergency use license (EUA) to Gilead Sciences Inc. for emergency use of remdesivir for the treatment of COVID-19 patients.
- In June 2020, the pharmaceutical company Mylan NV announced that it has received approval from the Indian Drug Administration DCGI to manufacture and market its drugs for limited emergency use in the country for the treatment of COVID-19.
- In May 2020, Hyderbad-based pharmaceutical companies Hetero and Cipla entered into exclusive licensing agreements with US pharmaceutical company Gilead Sciences Inc. for the manufacture and distribution of Remdesivir. Apart from Hetero and Cipla, Gilead also entered into an agreement with Jubilant Life Sciences and Mylan NV for the production and sale of Remdesivir in India.
With the increasing demand and increasing sale of remdesivir drugs, are fueling the growth of remdesivir drugs market in the near future.
As such, market participants are involved in the production of remdesivir drugs expanding their business through a variety of programs, including collaboration, contracts, and pipeline development, and market expansion. It is expected that the strategic decisions of these companies will provide significant opportunities for market participants operating in the remdesivir market.
CONCLUSION
Remdesivir is a new antiviral drug developed by Gilead Sciences, originally for the treatment of Ebola virus and Marburg virus infections. Remdesivir has broad-spectrum efficacy against members of several viral families, including filoviruses (for instance, Ebola) and coronaviruses (for instance, SARS-CoV and Middle Eastern Respiratory Syndrome (MERSCoV)], and has shown prophylactic and therapeutic efficacy in clinical trials of these chronic varicose veins. Intravenous remdesivir treatment showed significant improvement for the first case of COVID-19 in the United States, and since then a rapid study has been initiated to evaluate the efficacy and safety of remdesivir in patients admitted to hospital with 2019-nCoV infection.
There is evidence to support the safety and tolerability of remdesivir in short-term treatment. However, more evidence is needed to assess the effects of long-term treatment. Given the limitations of the evidence and the particular safety concerns that remain, the widespread use of remdesivir against pandemic COVID-19 should be justified.
Various manufacturers have allowed their manufacturers to operate a small production of remdesivir at various production sites in safe areas around the world, helping them to maintain a stable supply chain. In addition, increased demand for remdesivir for the treatment of coronavirus has increased profits.