The global pharmacogenetic testing market includes services that examine a person's genetic profile to ascertain how a person’s genes affect the reaction of the same to particular drugs. Specific genetic variations that may affect the metabolism, effectiveness, and potential side effects of different medications are identified by this specialized genetic testing. Healthcare professionals can customize drug selections and dosages for a patient to improve treatment outcomes, reduce side effects, and increase overall drug efficacy. This is done by being aware of that patient's genetic predispositions. The quality of healthcare and patient outcomes may improve due to this personalized medication selection and dosage approach.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-pharmacogenetic-testing-market
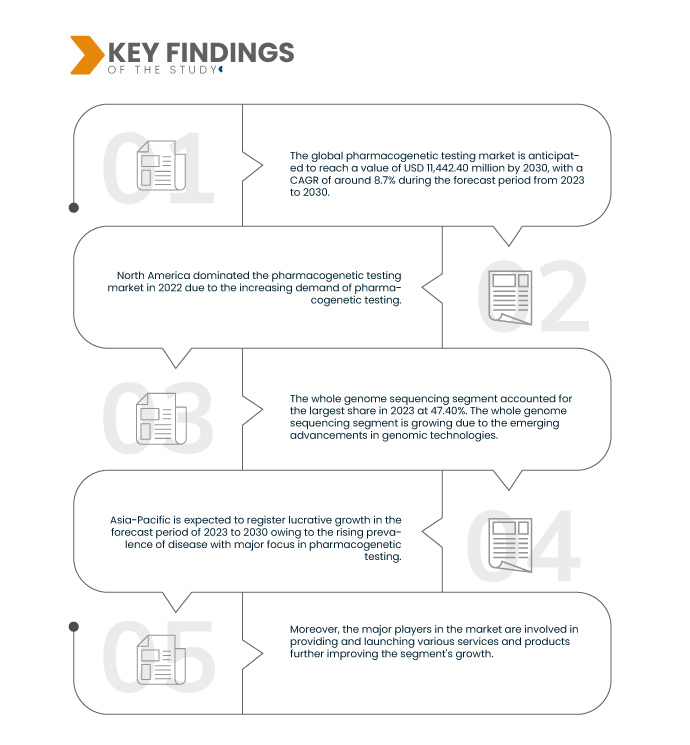
Data Bridge Market Research analyzes that the Global Pharmacogenetic Testing Market is expected to grow at a CAGR of 8.7% in the forecast period of 2023 to 2030 and is expected to reach USD 11,442.40 million by 2030. The sub-orbital pharmacogenetic testing segment is projected to propel the market growth as they are widely used and less complicated than others.
Key Findings of the Study
Advancements in Genomic Technologies
The ongoing surge in genomic innovation has significantly enhanced the precision, speed, and cost-effectiveness of genetic testing. Next-Generation Sequencing (NGS) and other cutting-edge molecular diagnostic techniques have emerged as pivotal tools, revolutionizing how genetic information is analyzed and interpreted. The integration of these advanced genomic technologies has bolstered the accuracy and scope of pharmacogenetic testing, allowing for comprehensive assessment of an individual's genetic profile. This, in turn, empowers healthcare professionals to make informed decisions regarding drug selection, dosing, and treatment strategies tailored to a patient's unique genetic makeup.
Moreover, CRISPR-Cas9 gene editing technology has shown promise in studying the functional impact of specific genetic variants. This technology allows researchers to precisely modify genes, mimicking certain genetic variations and elucidating their effects on drug metabolism and response. These advancements collectively contribute to the refinement and expansion of the market, bringing closer to a future where medications are tailored to each individual based on their unique genetic blueprint, which is expected to drive market growth.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Year
|
2021 (Customizable 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
Type (Whole Genome Sequencing, Whole Exome Sequencing, Array-Based Tests, and Single Gene Tests), Gene Type (CYP2C19, CYP2D6, CYP2C9 and VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX and MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT, and Others), Drug Type (Prescription Drugs, Nutraceuticals, Recreational Drugs, Herbal Supplements, Vitamins, and Over-the-Counter Medications), Sample (Blood and Saliva), Therapeutic Area (Cardiology, Gastroenterology, Anesthesiology, Genomics, Endocrinology, Immunology & Hypersensitivity, Dermatology, Gynecology, Oncology, Neurology, and Others), Application (Clinical Practice, Drug Development, and Drug Regulation), End User (Healthcare Providers, Pharmaceutical & Biotechnology Companies, Research Centres and Academic Institutes, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacies, Mail-Order Pharmacies, and Direct-to-Customer Services)
|
|
Countries Covered
|
U.S., Canada, Mexico, France, Germany, Spain, Italy, U.K., Spain, Netherlands, Russia, Turkey, Switzerland, Rest of Europe, China, Japan, Thailand, India, South Korea, Australia, Singapore, Indonesia, Philippines, Malaysia, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, U.A.E., Saudi Arabia, South Africa, Israel, Egypt, and Rest of Middle East and Africa
|
|
Market Players Covered
|
Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Sonic Healthcare (Australia), QIAGEN (U.S.), BGI (China), PerkinElmer Inc. (U.S.), Dynamic DNA Laboratories (U.S.), Eurofins Scientific (U.S.), 23andMe, Inc (U.S.), OneOme (U.S.), PacBio (U.S.), MD Labs (U.S.), GENEWIZ (U.S.), PGXT (U.S.), Luminex Corporation (U.S.), and Myriad Genetics, Inc. (U.S.) among others.
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The global pharmacogenetic testing market is segmented into eight notable segments based on type, gene type, drug type, sample, therapeutic area, application, end user, and distribution channel.
- On the basis of type, the market is segmented into whole genome sequencing, whole exome sequencing, array-based tests, and single gene tests.
In 2023, the whole genome sequencing segment of the type segment is expected to dominate the global pharmacogenetic testing market.
In 2023, the whole genome sequencing segment is expected to dominate the market with a market share of 47.40% due to higher demand and applicability in the pharmacogenomics sector.
- On the basis of gene type, the market is segmented into CYP2C19, CYP2D6, CYP2C9 and VKORC1, CYP1A2, HLA-B*1502, HLA-B*5701, CYP2D, OPRM1, ONCOTYPE DX and MAMMAPRINT, DRD3, D4D4, SLC6A4, HTR2A/C, TMPT, and others.
In 2023, the CYP2C19 segment of the gene type segment is expected to dominate the global pharmacogenetic testing market.
In 2023, the CYP2C19 segment is expected to dominate the market with 15.59% market share as this gene encodes an enzyme that plays a crucial role in the metabolism of a variety of drugs, including several commonly prescribed medications such as clopidogrel (antiplatelet) and omeprazole (proton pump inhibitor).
- On the basis of drug type, the market is segmented into Prescription Drugs, nutraceuticals, recreational drugs, Herbal Supplements, vitamins, and over-the-counter medications. In 2023, the prescription drugs segment is expected to dominate the market with a market share of 31.06%.
- On the basis of sample, the market is segmented into blood and saliva. In 2023, the blood segment is expected to dominate the market with a market share of 54.27%.
- On the basis of therapeutic area, the market is segmented into cardiology, gastroenterology, anesthesiology, Genomics, endocrinology, immunology & hypersensitivity, dermatology, gynecology, oncology, neurology, and others. In 2023, the cardiology segment is expected to dominate the market with a market share of 18.82%.
- On the basis of application, the market is segmented into clinical practice, drug development, and drug regulation. In 2023, the clinical practice segment is expected to dominate the market with a market share of 44.74%.
- On the basis of end user, the market is segmented into healthcare providers, pharmaceutical & biotechnology companies, research centers & academic institutes and others. In 2023, the healthcare providers segment is expected to dominate the market with a market share of 39.97%
- On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacies, mail-order pharmacies, and direct-to-customer services. In 2023, the hospital pharmacy segment is expected to dominate the market with a market share of 44.98%.
Major Players
Data Bridge Market Research analyzes Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Sonic Healthcare (Australia), QIAGEN (U.S.), BGI (China) as the major market players of the market.
Market Development
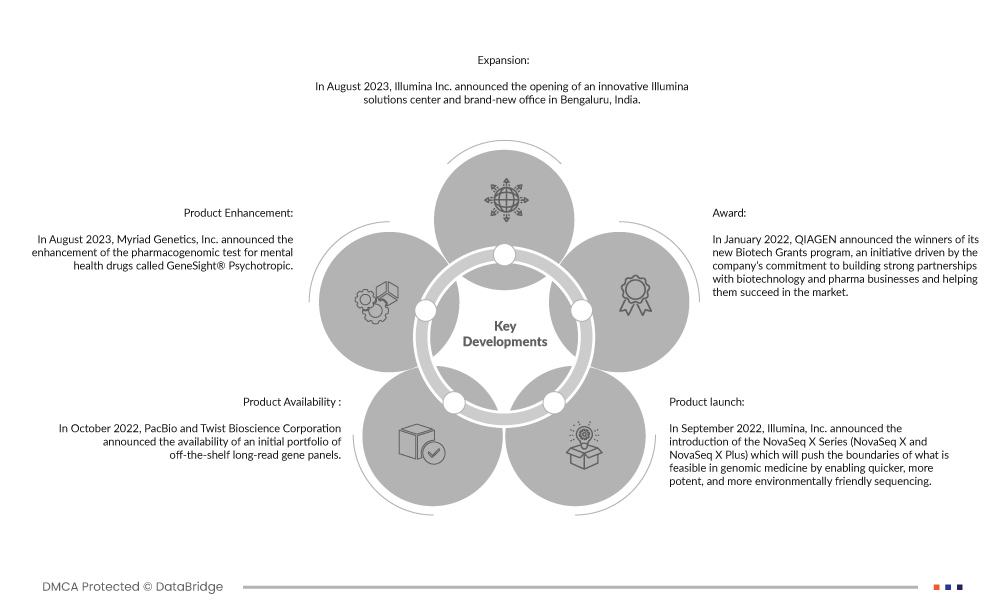
- In August 2023, Illumina Inc. announced the opening of an innovative Illumina solutions center and brand-new office in Bengaluru, India. With its permanent facility now, Illumina will keep working with Premas to expand the genomics market in India, which the UN considers to be the world's most populous nation with more than 1.4 billion inhabitants.
- In August 2023, Myriad Genetics, Inc. announced the enhancement of the pharmacogenomic test for mental health drugs called GeneSight® Psychotropic. The GeneSight report will now contain details on how a patient's smoking history could affect how their body processes particular drugs.
- In October 2022, PacBio and Twist Bioscience Corporation announced the availability of an initial portfolio of off-the-shelf long-read gene panels. These fixed Twist Alliance panels are made to efficiently and cost-effectively capture target areas. In addition, customers will be able to create a panel of their design that is fully adaptable and scalable for sequencing using PacBio HiFi reads.
- In September 2022, Illumina, Inc. announced the introduction of the NovaSeq X Series (NovaSeq X and NovaSeq X Plus), which will push the boundaries of what is feasible in genomic medicine by enabling quicker, more potent, and more environmentally friendly sequencing. With the aid of ground-breaking new technology, the NovaSeq X Plus can produce over 20,000 whole genomes annually - 2.5 times more numerous than previous sequencers - dramatically advancing genomic research and clinical understanding, ultimately revolutionizing patient lives.
- In January 2022, QIAGEN announced the winners of its new Biotech Grants program, an initiative driven by the company’s commitment to building strong partnerships with biotechnology and pharma businesses and helping them succeed in the market.
Regional Analysis
Geographically, the countries covered in the global pharmacogenetic testing market report are the U.S., Canada, Mexico, France, Germany, Spain, Italy, U.K., Spain, Netherlands, Russia, Turkey, Switzerland, Rest of Europe, China, Japan, Thailand, India, South Korea, Australia, Singapore, Indonesia, Philippines, Malaysia, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, U.A.E., Saudi Arabia, South Africa, Israel, Egypt, and Rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
North America is the dominant region in the global pharmacogenetic testing market.
North America is expected to dominate the market owing to the higher investments by U.S. manufacturers and service providers and the increasing advancement in genomic technologies. North America will continue to dominate the market in terms of market share and revenue and flourish its dominance during the forecast period.
Asia-Pacific is estimated to be the fastest-growing region in the global pharmacogenetic testing market during the forecast period of 2023 to 2030.
Asia-Pacific is expected to grow during the forecast period due to the growing population with the rising prevalence of chronic and complex diseases in this region.
For more detailed information about the global pharmacogenetic testing market report, click here – https://www.databridgemarketresearch.com/reports/global-pharmacogenetic-testing-market