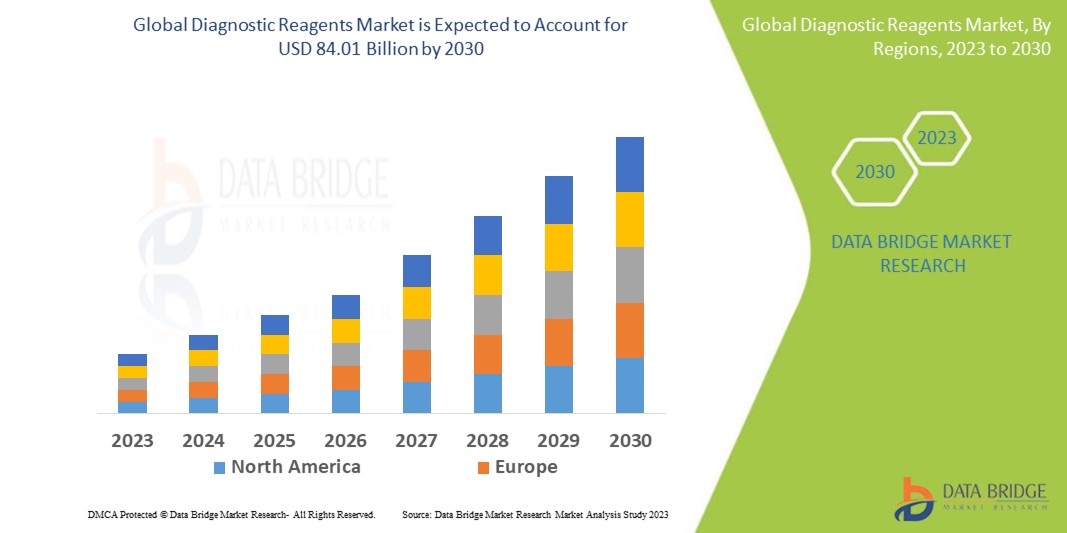
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL DIAGNOSTIC REAGENTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL DIAGNOSTIC REAGENTS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL DIAGNOSTIC REAGENTS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY TYPE
16.1 OVERVIEW
16.2 CHROMATOGRAPHY REAGENTS
16.3 MOLECULAR DIAGNOSTIC REAGENTS
16.4 IMMUNOASSAY REAGENTS
16.5 CLINICAL CHEMISTRY REAGENTS
16.6 FLOW CYTOMETRY REAGENTS
16.7 CELL AND TISSUE CULTURE REAGENTS
16.8 HEMATOLOGY AND HEMOSTASIS REAGENTS
16.9 MICROBIOLOGY REAGENTS
16.1 OTHERS
17 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY PRODUCTS
17.1 OVERVIEW
17.2 MOLECULAR DIAGNOSTIC REAGENTS
17.2.1 NUCLEIC ACID EXTRACTION REAGENTS
17.2.1.1. DNA EXTRACTION REAGENTS
17.2.1.2. RNA EXTRACTION REAGENTS
17.2.2 POLYMERASE CHAIN REACTION (PCR) REAGENTS
17.2.2.1. DNA POLYMERASE
17.2.2.2. PRIMERS
17.2.2.3. NUCLEOTIDE MIX
17.2.2.4. BUFFER SOLUTIONS
17.2.2.5. MGCL2
17.2.2.6. OTHERS
17.2.3 REVERSE TRANSCRIPTION REAGENTS
17.2.3.1. REVERSE TRANSCRIPTASE
17.2.3.2. RANDOM PRIMERS OR OLIGO(DT)
17.2.4 QUANTITATIVE PCR (QPCR) REAGENTS
17.2.4.1. FLUORESCENT PROBES
17.2.4.2. REFERENCE STANDARDS
17.2.4.3. OTHERS
17.2.5 DNA/RNA LABELING AND DETECTION
17.2.5.1. FLUORESCENT DYES OR RADIOACTIVE PROBES
17.2.5.2. CHEMILUMINESCENT SUBSTRATES
17.2.5.3. OTHERS
17.2.6 NEXT-GENERATION SEQUENCING (NGS) REAGENTS
17.2.6.1. SEQUENCING REAGENTS
17.2.6.2. INDEXING PRIMERS
17.2.6.3. OTHERS
17.2.7 PROTEIN DETECTION REAGENTS
17.2.8 ISOTHERMAL AMPLIFICATION REAGENTS
17.2.9 CRISPR-CAS REAGENTS
17.2.9.1. GUIDE RNA (GRNA)
17.2.9.2. CAS PROTEIN
17.2.9.3. OTHERS
17.2.10 HYBRIDIZATION AND MICROARRAY REAGENTS
17.2.10.1. CAPTURE PROBES
17.2.10.2. DETECTION PROBES
17.2.10.3. OTHERS
17.3 IMMUNOASSAY REAGENTS
17.3.1 ANTIBODIES
17.3.1.1. PRIMARY ANTIBODIES
17.3.1.2. SECONDARY ANTIBODIES
17.3.2 ENZYMES
17.3.2.1. HORSERADISH PEROXIDASE (HRP)
17.3.2.2. ALKALINE PHOSPHATASE (AP)
17.3.3 FLUOROPHORES
17.3.3.1. FLUORESCEIN ISOTHIOCYANATE (FITC)
17.3.3.2. RHODAMINE
17.3.3.3. ALEXA FLUOR DYES
17.3.3.4. OTHERS
17.3.4 CHEMILUMINESCENT SUBSTRATES
17.3.5 BIOTINYLATION REAGENTS
17.3.6 BLOCKING REAGENTS
17.3.7 CALIBRATORS AND CONTROLS
17.3.7.1. CALIBRATION STANDARDS
17.3.7.2. POSITIVE AND NEGATIVE CONTROLS
17.3.8 BUFFER SOLUTIONS
17.3.8.1. PBS (PHOSPHATE-BUFFERED SALINE)
17.3.8.2. TRIS-HCL
17.3.8.3. OTHERS
17.3.9 RECOMBINANT OR PURIFIED ANTIGENS
17.3.10 WASH BUFFERS
17.3.11 OTHERS
17.4 CLINICAL CHEMISTRY REAGENTS
17.4.1 ENZYMES
17.4.1.1. ALANINE AMINOTRANSFERASE (ALT) REAGENTS
17.4.1.2. ASPARTATE AMINOTRANSFERASE (AST) REAGENTS
17.4.1.3. ALKALINE PHOSPHATASE (ALP) REAGENTS
17.4.1.4. OTHERS
17.4.2 SUBSTRATES
17.4.2.1. GLUCOSE REAGENTS
17.4.2.2. OTHERS
17.4.3 LIPID PROFILE REAGENTS
17.4.3.1. CHOLESTEROL REAGENTS
17.4.3.2. TRIGLYCERIDE REAGENTS
17.4.3.3. HIGH-DENSITY LIPOPROTEIN (HDL) CHOLESTEROL REAGENTS
17.4.3.4. LOW-DENSITY LIPOPROTEIN (LDL) CHOLESTEROL REAGENTS
17.4.4 ELECTROLYTES
17.4.4.1. SODIUM
17.4.4.2. POTASSIUM
17.4.4.3. CHLORIDE REAGENTS
17.4.4.4. OTHERS
17.4.5 KIDNEY FUNCTION REAGENTS
17.4.5.1. BLOOD UREA NITROGEN (BUN) REAGENTS
17.4.5.2. CREATININE REAGENTS
17.4.5.3. OTHER
17.4.6 LIVER FUNCTION REAGENTS
17.4.6.1. BILIRUBIN REAGENTS
17.4.6.2. GAMMA-GLUTAMYL TRANSFERASE (GGT) REAGENTS
17.4.6.3. OTHERS
17.4.7 CARDIAC MARKERS
17.4.7.1. TROPONIN REAGENTS
17.4.7.2. CREATINE KINASE (CK) REAGENTS
17.4.7.3. OTHERS
17.4.8 HORMONES
17.4.8.1. THYROID FUNCTION REAGENTS
17.4.8.2. CORTISOL REAGENTS
17.4.8.3. OTHERS
17.4.9 PROTEINS
17.4.9.1. ALBUMIN REAGENTS
17.4.9.2. TOTAL PROTEIN REAGENTS
17.4.9.3. OTHERS
17.4.10 COAGULATION REAGENTS
17.4.10.1. PROTHROMBIN TIME (PT) REAGENTS
17.4.10.2. ACTIVATED PARTIAL THROMBOPLASTIN TIME (APTT) REAGENTS
17.4.10.3. OTHERS
17.4.11 URINE CHEMISTRY REAGENTS
17.4.11.1. URINE GLUCOSE REAGENTS
17.4.11.2. URINE PROTEIN REAGENTS
17.4.11.3. OTHERS
17.4.12 SPECIFIC PROTEINS
17.4.12.1. C-REACTIVE PROTEIN (CRP) REAGENTS
17.4.12.2. HEMOGLOBIN A1C (HBA1C) REAGENTS
17.4.12.3. OTHERS
17.5 FLOW CYTOMETRY REAGENTS
17.5.1 FLUOROCHROME-CONJUGATED ANTIBODIES
17.5.1.1. FLUORESCEIN ISOTHIOCYANATE (FITC)
17.5.1.2. PHYCOERYTHRIN (PE)
17.5.1.3. PERIDININ-CHLOROPHYLL PROTEIN (PERCP)
17.5.1.4. ALLOPHYCOCYANIN (APC)
17.5.1.5. ALEXA FLUOR DYES
17.5.1.6. OTHERS
17.5.2 CELL VIABILITY DYES
17.5.2.1. PROPIDIUM IODIDE (PI)
17.5.2.2. 7-AMINOACTINOMYCIN D (7-AAD)
17.5.2.3. FIXABLE VIABILITY DYES
17.5.2.4. OTHERS
17.5.3 NUCLEIC ACID STAINS
17.5.3.1. 4',6-DIAMIDINO-2-PHENYLINDOLE (DAPI)
17.5.3.2. HOECHST DYES
17.5.3.3. OTHERS
17.5.4 ISOTYPE CONTROLS
17.5.5 OTHERS
17.6 CELL AND TISSUE CULTURE REAGENTS
17.6.1 CELL CULTURE MEDIA
17.6.1.1. DULBECCO'S MODIFIED EAGLE MEDIUM (DMEM)
17.6.1.2. RPMI 1640
17.6.1.3. MINIMUM ESSENTIAL MEDIUM (MEM)
17.6.1.4. OTHERS
17.6.2 SERUM
17.6.2.1. FETAL BOVINE SERUM (FBS)
17.6.2.2. BOVINE SERUM ALBUMIN (BSA)
17.6.2.3. OTHERS
17.6.3 ANTIBIOTICS AND ANTIMYCOTICS
17.6.3.1. PENICILLIN-STREPTOMYCIN
17.6.3.2. AMPHOTERICIN B
17.6.3.3. OTHERS
17.6.4 TRYPSIN AND TRYPSIN INHIBITORS
17.6.4.1. TRYPSIN
17.6.4.2. TRYPSIN-EDTA
17.6.4.3. TRYPSIN INHIBITOR
17.6.4.4. OTHERS
17.6.5 CELL DISSOCIATION REAGENTS
17.6.5.1. ACCUTASE
17.6.5.2. COLLAGENASE
17.6.5.3. DISPASE
17.6.5.4. OTHERS
17.6.6 GROWTH FACTORS AND CYTOKINES
17.6.7 CELL FREEZING AND THAWING REAGENTS
17.6.7.1. DMSO (DIMETHYL SULFOXIDE)
17.6.7.2. CRYOPROTECTIVE AGENTS
17.6.7.3. OTHERS
17.6.8 EXTRACELLULAR MATRIX PROTEINS
17.6.8.1. COLLAGEN
17.6.8.2. FIBRONECTIN
17.6.8.3. LAMININ
17.6.8.4. OTHERS
17.6.9 HYBRIDOMA CULTURE REAGENTS
17.6.10 OTHERS
17.7 HEMATOLOGY AND HEMOSTASIS REAGENTS
17.7.1 HEMATOLOGY REAGENTS
17.7.1.1. HEMATOLOGY STAINS
17.7.1.1.1. WRIGHT-GIEMSA STAIN
17.7.1.1.2. NEW METHYLENE BLUE STAIN
17.7.1.2. CBC REAGENTS
17.7.1.2.1. DILUENT
17.7.1.2.2. LYSING REAGENTS
17.7.1.2.3. OTHERS
17.7.1.3. AUTOMATED HEMATOLOGY ANALYZER REAGENTS
17.7.1.3.1. HEMATOLOGY CONTROLS
17.7.1.3.2. CALIBRATION STANDARDS
17.7.1.3.3. OTHERS
17.7.1.4. MANUAL CELL COUNTING REAGENTS
17.7.1.5. HEMOGLOBIN REAGENTS
17.7.1.6. ERYTHROCYTE SEDIMENTATION RATE (ESR) REAGENTS
17.7.1.7. OTHERS
17.7.2 HEMOSTASIS REAGENTS
17.7.2.1. PROTHROMBIN TIME (PT) REAGENTS
17.7.2.2. THROMBOPLASTIN REAGENTS
17.7.2.3. ACTIVATED PARTIAL THROMBOPLASTIN TIME (APTT) REAGENTS
17.7.2.4. CLAUSS METHOD REAGENTS
17.7.2.5. ANTICOAGULANT MONITORING REAGENTS
17.7.2.6. OTHERS
17.8 MICROBIOLOGY REAGENTS
17.8.1 BIOCHEMICAL TEST REAGENTS
17.8.1.1. TRIPLE SUGAR IRON (TSI)
17.8.1.2. SIMMONS' CITRATE AGAR
17.8.1.3. UREA AGAR
17.8.1.4. OTHERS
17.8.2 SEROLOGICAL REAGENTS
17.8.2.1. ANTIBODY REAGENTS
17.8.2.2. ANTIGEN DETECTION REAGENTS
17.8.2.3. OTHERS
17.8.3 ANTIMICROBIAL SUSCEPTIBILITY TESTING (AST) REAGENTS
17.8.4 MYCOLOGY REAGENTS
17.8.4.1. SABOURAUD DEXTROSE AGAR (SDA)
17.8.4.2. LACTOPHENOL COTTON BLUE STAIN
17.8.4.3. OTHERS
17.8.5 MICROBIAL PRESERVATION REAGENTS
17.8.6 LYOPHILIZATION REAGENTS
17.8.7 OTHERS
17.9 CHROMATOGRAPHY REAGENTS
17.1 OTHERS
18 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY NATURE
18.1 OVERVIEW
18.2 CHEMICAL REAGENTS
18.3 BIOLOGICAL REAGENTS
19 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 INFECTIOUS DISEASE TESTING
19.2.1 HIV
19.2.2 HEPATITIS
19.2.3 TUBERCULOSIS
19.2.4 COVID-19
19.2.5 OTHERS
19.3 ONCOLOGY
19.3.1 CANCER BIOMARKERS
19.3.2 GENETIC TESTING
19.3.3 OTHERS
19.4 CARDIOLOGY
19.4.1 CARDIAC MARKERS
19.4.2 LIPID PROFILES
19.4.3 OTHERS
19.5 DIABETES:
19.5.1 GLUCOSE TESTING
19.5.2 HBA1C TESTING
19.5.3 OTHERS
19.6 HEMATOLOGY
19.6.1 COMPLETE BLOOD COUNT (CBC)
19.6.2 COAGULATION TESTS
19.6.3 OTHERS
19.7 OTHERS
20 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 DIAGNOSTIC CENTER
20.4 RESEARCH LABS & INSTITUTES
20.5 BLOOD BANKS
20.6 SPECILATY CLINICS
20.7 AMBULATORY SURGICAL CENTERS (ASCS)
20.8 OTHERS
21 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDERS
21.3 RETAIL SALES
21.4 ONLINE SALES
21.5 OTHERS
22 GLOBAL DIAGNOSTIC REAGENTS MARKET, COMPANY LANDSCAPE
22.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
22.2 COMPANY SHARE ANALYSIS: EUROPE
22.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
22.4 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
22.5 COMPANY SHARE ANALYSIS: SOUTH AMERICA
22.6 MERGERS & ACQUISITIONS
22.7 NEW PRODUCT DEVELOPMENT & APPROVALS
22.8 EXPANSIONS
22.9 REGULATORY CHANGES
22.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
23 GLOBAL DIAGNOSTIC REAGENTS MARKET, BY REGION
GLOBAL DIAGNOSTIC REAGENTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 NORTH AMERICA
23.1.1 U.S.
23.1.2 CANADA
23.1.3 MEXICO
23.2 EUROPE
23.2.1 GERMANY
23.2.2 FRANCE
23.2.3 U.K.
23.2.4 HUNGARY
23.2.5 LITHUANIA
23.2.6 AUSTRIA
23.2.7 IRELAND
23.2.8 NORWAY
23.2.9 POLAND
23.2.10 ITALY
23.2.11 SPAIN
23.2.12 RUSSIA
23.2.13 TURKEY
23.2.14 BELGIUM
23.2.15 NETHERLANDS
23.2.16 SWITZERLAND
23.2.17 REST OF EUROPE
23.3 ASIA-PACIFIC
23.3.1 JAPAN
23.3.2 CHINA
23.3.3 SOUTH KOREA
23.3.4 INDIA
23.3.5 AUSTRALIA
23.3.6 SINGAPORE
23.3.7 THAILAND
23.3.8 MALAYSIA
23.3.9 INDONESIA
23.3.10 PHILIPPINES
23.3.11 NEW ZEALAND
23.3.12 TAIWAN
23.3.13 VIETNAM
23.3.14 REST OF ASIA-PACIFIC
23.4 SOUTH AMERICA
23.4.1 BRAZIL
23.4.2 ARGENTINA
23.4.3 PERU
23.4.4 REST OF SOUTH AMERICA
23.5 MIDDLE EAST AND AFRICA
23.5.1 SOUTH AFRICA
23.5.2 SAUDI ARABIA
23.5.3 UAE
23.5.4 EGYPT
23.5.5 KUWAIT
23.5.6 ISRAEL
23.5.7 REST OF MIDDLE EAST AND AFRICA
23.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL DIAGNOSTIC REAGENTS MARKET, SWOT AND DBMR ANALYSIS
25 GLOBAL DIAGNOSTIC REAGENTS MARKET, COMPANY PROFILE
25.1 THERMO FISHER SCIENTIFIC
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 QUIDELORTHO CORPORATION
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 ILLUMINA, INC.
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 MERCK KGAA,
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 PERKINELMER INC
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 SHIMADZU CORPORATION
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 HOLOGIC, INC.
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 CYTENA GMBH
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 ABBOTT
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 BIO-RAD LABORATORIES, INC.
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 DANAHER
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 BIOMÉRIEUX
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 QIAGEN
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 GRIFOLS, S.A.
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 FUJIREBIO DIAGNOSTICS AB
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 MERIDIAN BIOSCIENCE
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 EKF DIAGNOSTICS HOLDINGS PLC
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 RANDOX LABORATORIES LTD.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 SEKISUI DIAGNOSTICS
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 BD
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 SIEMENS HEALTHCARE GMBH
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
25.22 F. HOFFMANN-LA ROCHE LTD
25.22.1 COMPANY OVERVIEW
25.22.2 REVENUE ANALYSIS
25.22.3 GEOGRAPHIC PRESENCE
25.22.4 PRODUCT PORTFOLIO
25.22.5 RECENT DEVELOPMENTS
25.23 DIASORIN S.P.A.
25.23.1 COMPANY OVERVIEW
25.23.2 REVENUE ANALYSIS
25.23.3 GEOGRAPHIC PRESENCE
25.23.4 PRODUCT PORTFOLIO
25.23.5 RECENT DEVELOPMENTS
25.24 SYSMEX CORPORATION
25.24.1 COMPANY OVERVIEW
25.24.2 REVENUE ANALYSIS
25.24.3 GEOGRAPHIC PRESENCE
25.24.4 PRODUCT PORTFOLIO
25.24.5 RECENT DEVELOPMENTS
25.25 ORTHO CLINICAL DIAGNOSTICS
25.25.1 COMPANY OVERVIEW
25.25.2 REVENUE ANALYSIS
25.25.3 GEOGRAPHIC PRESENCE
25.25.4 PRODUCT PORTFOLIO
25.25.5 RECENT DEVELOPMENTS
25.26 AGILENT TECHNOLOGIES INC.
25.26.1 COMPANY OVERVIEW
25.26.2 REVENUE ANALYSIS
25.26.3 GEOGRAPHIC PRESENCE
25.26.4 PRODUCT PORTFOLIO
25.26.5 RECENT DEVELOPMENTS
25.27 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD.
25.27.1 COMPANY OVERVIEW
25.27.2 REVENUE ANALYSIS
25.27.3 GEOGRAPHIC PRESENCE
25.27.4 PRODUCT PORTFOLIO
25.27.5 RECENT DEVELOPMENTS
25.28 FUJIFILM
25.28.1 COMPANY OVERVIEW
25.28.2 REVENUE ANALYSIS
25.28.3 GEOGRAPHIC PRESENCE
25.28.4 PRODUCT PORTFOLIO
25.28.5 RECENT DEVELOPMENTS
25.29 BIOSINO BIO-TECHNOLOGY AND SCIENCE INC.
25.29.1 COMPANY OVERVIEW
25.29.2 REVENUE ANALYSIS
25.29.3 GEOGRAPHIC PRESENCE
25.29.4 PRODUCT PORTFOLIO
25.29.5 RECENT DEVELOPMENTS
25.3 DAAN GENE CO., LTD.
25.30.1 COMPANY OVERVIEW
25.30.2 REVENUE ANALYSIS
25.30.3 GEOGRAPHIC PRESENCE
25.30.4 PRODUCT PORTFOLIO
25.30.5 RECENT DEVELOPMENTS
25.31 HIPRO BIOTECHNOLOGY CO.,LTD.
25.31.1 COMPANY OVERVIEW
25.31.2 REVENUE ANALYSIS
25.31.3 GEOGRAPHIC PRESENCE
25.31.4 PRODUCT PORTFOLIO
25.31.5 RECENT DEVELOPMENTS
25.32 TRIVITRON HEALTHCARE
25.32.1 COMPANY OVERVIEW
25.32.2 REVENUE ANALYSIS
25.32.3 GEOGRAPHIC PRESENCE
25.32.4 PRODUCT PORTFOLIO
25.32.5 RECENT DEVELOPMENTS
25.33 DIAGNOSTIC SYSTEMS GMBH
25.33.1 COMPANY OVERVIEW
25.33.2 REVENUE ANALYSIS
25.33.3 GEOGRAPHIC PRESENCE
25.33.4 PRODUCT PORTFOLIO
25.33.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH