1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
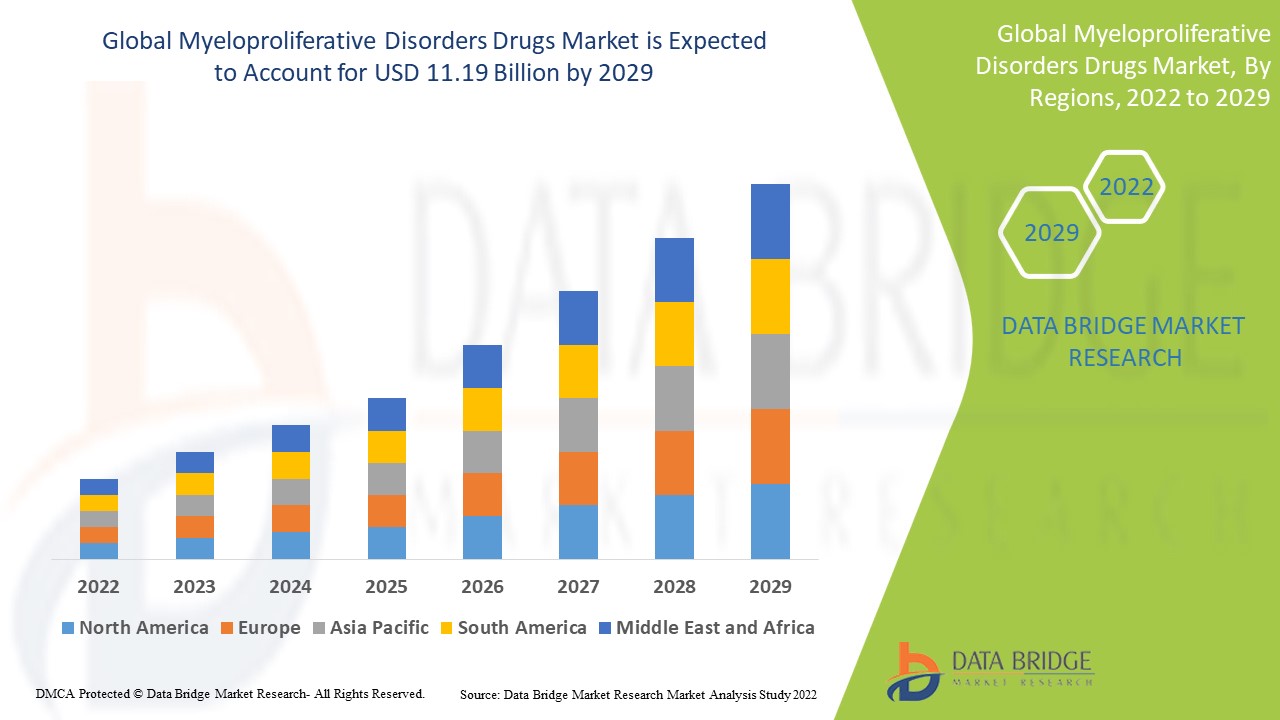
1.3 OVERVIEW OF GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL XX SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY TYPE
14.1 OVERVIEW
14.2 PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
14.2.1 ESSENTIAL THROMBOCYTHEMIA (ET)
14.2.2 POLYCYTHEMIA VERA (PV)
14.2.3 IDIOPATHIC MYELOFIBROSIS (IMF)
14.3 PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
14.3.1 BY PHASES
14.3.1.1. CHRONIC PHASE
14.3.1.2. ACCELERATED PHASE
14.3.1.3. BLAST PHASE
15 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY TREATMENT TYPE
15.1 OVERVIEW
15.2 TARGETED THERAPY
15.2.1 JAK INHIBITORS
15.2.1.1. RUXOLITINIB
15.2.1.2. FEDRATINIB
15.2.2 TYROSINE KINASE INHIBITOR
15.2.2.1. IMATINIB
15.2.2.2. NILOTINIB
15.2.2.3. BOSUTINIB
15.2.2.4. DASATINIB
15.2.2.5. BAFETINIB
15.2.2.6. PACRITINIB
15.2.2.7. OTHERS
15.2.3 THALIDOMIDE
15.2.4 OTHERS
15.3 CHEMOTHERAPY
15.3.1 HYDROXYUREA
15.3.2 CYTARABINE
15.3.3 ANTHRACYCLINES
15.3.4 ALKYLATING AGENTS
15.3.4.1. BUSULFAN
15.3.4.2. MELPHALAN
15.3.5 AZACITIDINE
15.3.6 OTHERS
15.4 OTHERS
15.4.1 ANAGRELIDE
15.4.2 DANAZOL
15.4.3 LENALIDOMIDE
15.4.4 INTERFERON ALPHA
15.4.5 ARSENIC TRIOXIDE
15.4.6 OTHERS
16 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY ROUTE OF ADMINISTRATION
16.1 OVERVIEW
16.2 ORAL
16.2.1 TABLETS
16.2.2 CAPSULES
16.3 PARENTERAL
16.3.1 INTRAMUSCULAR
16.3.2 INTRAVENOUS
16.3.3 SUBCUTANEOUS
16.3.4 OTHERS (IF ANY)
17 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 GLEEVEC
17.2.2 SPRYCEL
17.2.3 TASIGNA
17.2.4 JAKAFI
17.2.5 THALOMID
17.2.6 AGRYLIN
17.2.7 SIKLOS
17.2.8 INREBIC
17.2.9 DROXIA
17.2.10 SPRYCEL
17.2.11 OTHERS
17.3 GENERIC
18 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY MODE OF PURCHASE
18.1 OVERVIEW
18.2 PRESCRIPTION
18.3 OVER THE COUNTER (OTC)
19 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY POPULATION TYPE
19.1 OVERVIEW
19.2 PEDIATRIC
19.2.1 BY TYPE
19.2.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.2.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.2.1.1.2. POLYCYTHEMIA VERA (PV)
19.2.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.2.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
19.3 ADULTS
19.3.1 BY TYPE
19.3.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.3.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.3.1.1.2. POLYCYTHEMIA VERA (PV)
19.3.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.3.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
19.4 GERIATRIC
19.4.1 BY TYPE
19.4.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.4.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.4.1.1.2. POLYCYTHEMIA VERA (PV)
19.4.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.4.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
20 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.2.1 BY TYPE
20.2.1.1. PRIVATE
20.2.1.2. PUBLIC
20.3 SPECIALTY CLINICS
20.4 DIAGNOSTICS CENTERS
20.5 RESEARCH INSTITUTES
20.6 HOMECARE SETTINGS
20.7 OTHERS
21 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 HOSPITAL PHARMACY
21.3 ONLINE PHARMACY
21.4 RETAIL PHARMACY
22 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, SWOT AND DBMR ANALYSIS
23 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY REGION
24.1 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 NORTH AMERICA
24.2.1 U.S.
24.2.2 CANADA
24.2.3 MEXICO
24.3 EUROPE
24.3.1 GERMANY
24.3.2 U.K.
24.3.3 ITALY
24.3.4 FRANCE
24.3.5 SPAIN
24.3.6 RUSSIA
24.3.7 SWITZERLAND
24.3.8 TURKEY
24.3.9 BELGIUM
24.3.10 NETHERLANDS
24.3.11 DENMARK
24.3.12 SWEDEN
24.3.13 POLAND
24.3.14 NORWAY
24.3.15 FINLAND
24.3.16 REST OF EUROPE
24.4 ASIA-PACIFIC
24.4.1 JAPAN
24.4.2 CHINA
24.4.3 SOUTH KOREA
24.4.4 INDIA
24.4.5 SINGAPORE
24.4.6 THAILAND
24.4.7 INDONESIA
24.4.8 MALAYSIA
24.4.9 PHILIPPINES
24.4.10 AUSTRALIA
24.4.11 NEW ZEALAND
24.4.12 VIETNAM
24.4.13 TAIWAN
24.4.14 REST OF ASIA-PACIFIC
24.5 SOUTH AMERICA
24.5.1 BRAZIL
24.5.2 ARGENTINA
24.5.3 REST OF SOUTH AMERICA
24.6 MIDDLE EAST AND AFRICA
24.6.1 SOUTH AFRICA
24.6.2 EGYPT
24.6.3 BAHRAIN
24.6.4 UNITED ARAB EMIRATES
24.6.5 KUWAIT
24.6.6 OMAN
24.6.7 QATAR
24.6.8 SAUDI ARABIA
24.6.9 REST OF MEA
24.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, COMPANY PROFILE
25.1 PFIZER INC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 F. HOFFMANN-LA ROCHE LTD
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 NOVARTIS PHARMACEUTICALS CORPORATION
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 BRISTOL-MYERS SQUIBB
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 TAKEDA PHARMACEUITCAL COMPANY LIMITED
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 PHARACYCLICS LLC (AN ABBVIE COMPANY)
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 FRESENIUS KABI
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 HIKMA PHARMACEUTICALS PLC
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 TEVA PHARMACEUITCALS
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 MEDUNIK (SUBSIDIARY OF DUCHESNAY PHARMACEUTICAL GROUP INC.)
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 SUN PHARMACEUTICAL INDUSTRIES LTD.
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 SANOFI
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 CTI BIOPHARMA CORP.
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 PAR PHARMACEUITCAL
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 INCYTE
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH